Soluble Form of Carbonic Anhydrase IX (s-CA IX), Assays to Detect s-CA IX, CA IX's Coexpression with Her-2/neu/c-erbB-2, and CA IX-Specific Monoclonal Antibodies to Non-Immunodominant Epitopes
a carbonic anhydrase and carbon dioxide technology, applied in the field of medical genetics, can solve problems such as significant production obstacles, and achieve the effects of enlarge the clinical perspective, the therapeutic resource and diagnostic/prognostic parameters, and enhance the effectiveness of several chemotherapeutic agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
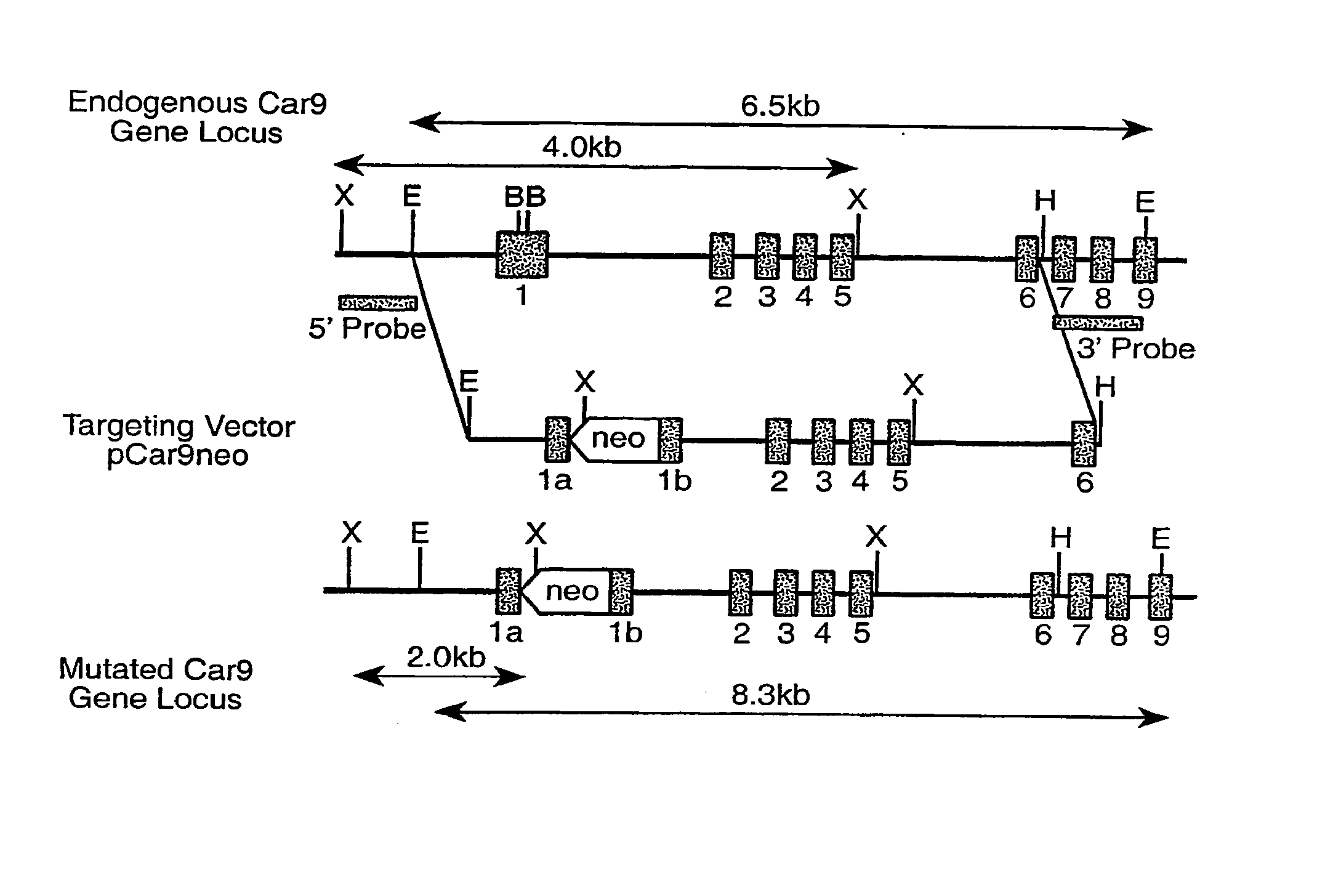
Preparation of Ca Ix-Deficient Mice
Materials and Methods
[0571]Cloning of Mouse Car9 cDNA
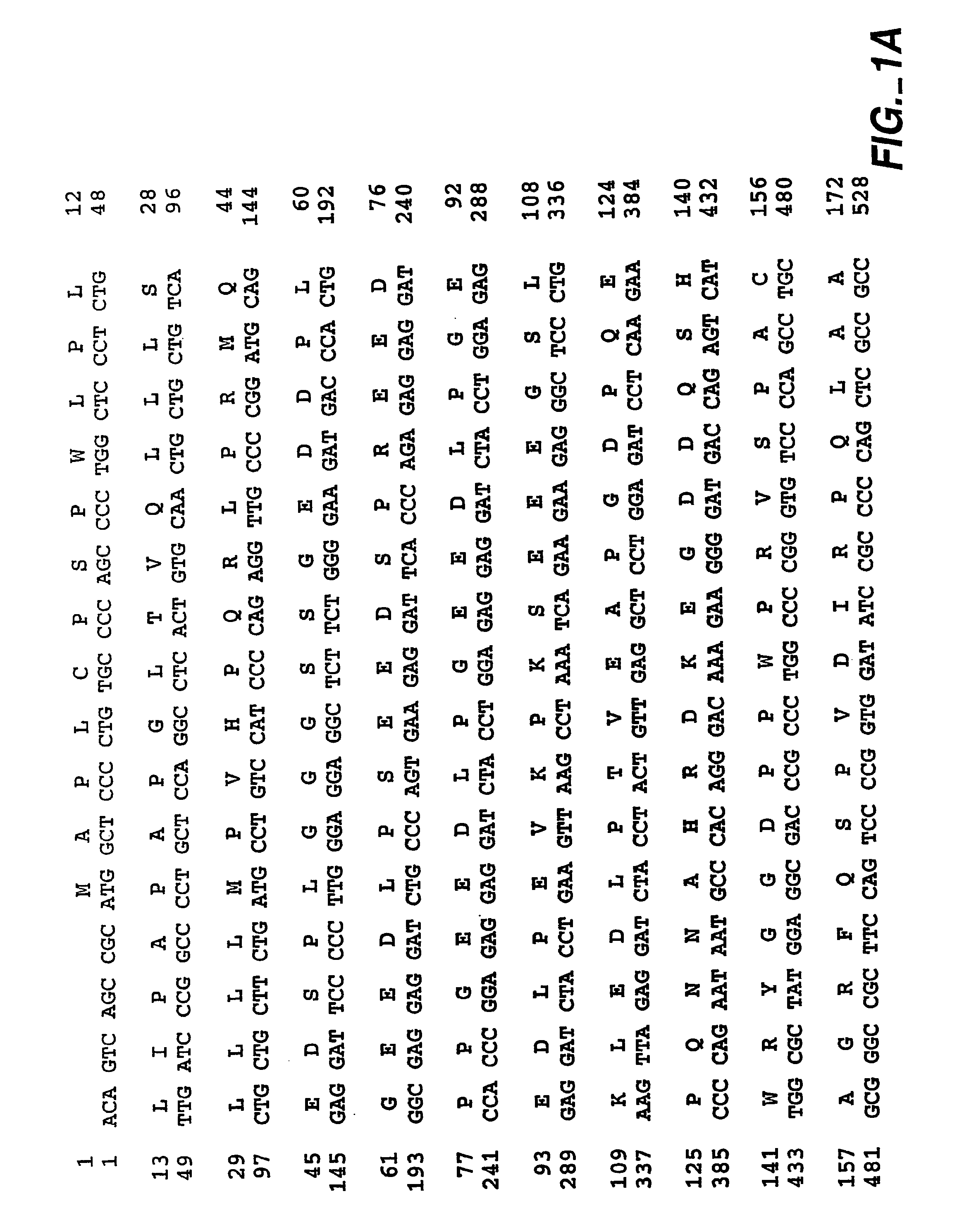
[0572]Mouse cDNA was obtained by RT PCR using primers derived from a human Car9 cDNA (EMBL Accession No. X66839; SEQ ID NO: 70). The template total RNA was prepared from C57BL / 6J mouse stomach by acid guanidium thiocyanate-phenol / chloroform isolation [Siebert et al. (1993)]. First strand synthesis was carried out under standard conditions using SuperScript II reverse transcriptase (Life Technologies, Germany). The set of the human primers was as follows: sense ATC CAC GTG GTT CAC CTC AG (SEQ ID NO: 71) and antisense CTTTGG TTC CCC TTC TGT GC (SEQ ID NO: 72); corresponding to the human cDNA fragment 760-1345 [SEQ ID NO: 73] of FIG. 1A-1C. PCR reactions were carried out in 30 cycles (94° C., 30 sec, 55° C., 40 sec, and 72° C., 60 sec). Entire cDNA including open reading frame, 3′- and 5′-untranslated regions, was obtained by the 3′ and 5′ RACE (rapid amplification of cDNA ends). The 3′ and 5′ RACE ...
example 2
CA IX-Specific Monoclonal Antibodies Generated from CA IX—Deficient Mice
Materials and Methods
[0627]Cell Culture
[0628]Hybridoma cell lines were grown in DMEM medium supplemented with 10% FCS (BioWhittaker, Verviers, Belgium), 2 mM glutamine and 40 μg / ml gentamicin (Lek, Slovenia) at 37° C. in 5% CO2 in air. The same cultivation conditions were applied to following cell lines that were used either as a source of CA IX antigen or as negative controls: mouse NIH 3T3 fibroblasts permanently transfected with the full-length human CA9 cDNA (NIH 3T3-flCA IX) and corresponding mock transfected NIH 3T3-neo controls [Pastorek et al. (1994)], MDCK cells transfected with the full-length CA9 cDNA (MDCK-flCA IX) and corresponding MDCK-neo controls, human HT 29 colon carcinoma cells as well as human HeLa cervical carcinoma cells naturally expressing CA IX, and C33a cervical carcinoma cells negative for CA IX.
[0629]For the immunization purposes, the cells grown for 2448 h were washed twice in PBS, s...
examples 3-10
[0675]The following materials and methods were used in the Examples 3-10 below.
Materials and Methods
Cell and Tumor Cultures and Media
[0676]The cell line HT29 (DSMZ ACC299), derived from colorectal carcinoma, and A498 (ATCC No. HTB-44) from renal clear cell carcinoma, were grown in D-MEM supplied with 10% FCS (Gibco), unless otherwise stated. In Example 8, the HT29 cells were cultivated in Dulbecco's MEM medium (Biowhiftaker, Verviers, Belgium) supplemented with 10% FCS (BioWhittaker) and 160 g / ml gentamicin (Lek, Slovenia). The A549 lung carcinoma cells were grown in DMEM (Sigma) supplemented with 10% FCS (Globepharm), L-glutamine (2 μM), penicillin (50 IU / ml), and streptomycin sulphate (50 μg / ml). Cell cultures were maintained in a humidified atmosphere with 5% CO2 at 37° C. For short-term cultures of tumors, fresh tumor excisions were cut in 1 mm pieces, rinsed with PBS, suspended in D-MEM with 10% FCS (about 50-100 mg of fresh weight of tumor fragments in 5 ml of the medium) and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| apparent molecular weight | aaaaa | aaaaa |
| apparent molecular weight | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com