Recombinant antibodies for treatment of respiratory syncytial virus infections
An antibody and virus technology, applied in the field of treatment or improvement of one or more symptoms related to RSV infection, prevention, polyclonal expression cell lines, can solve problems that hinder widespread application and inconvenience to patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0220] This example is a collection for illustrating the methods of the present invention.
[0221] a. Sorting lambda-negative plasmablasts from donor blood
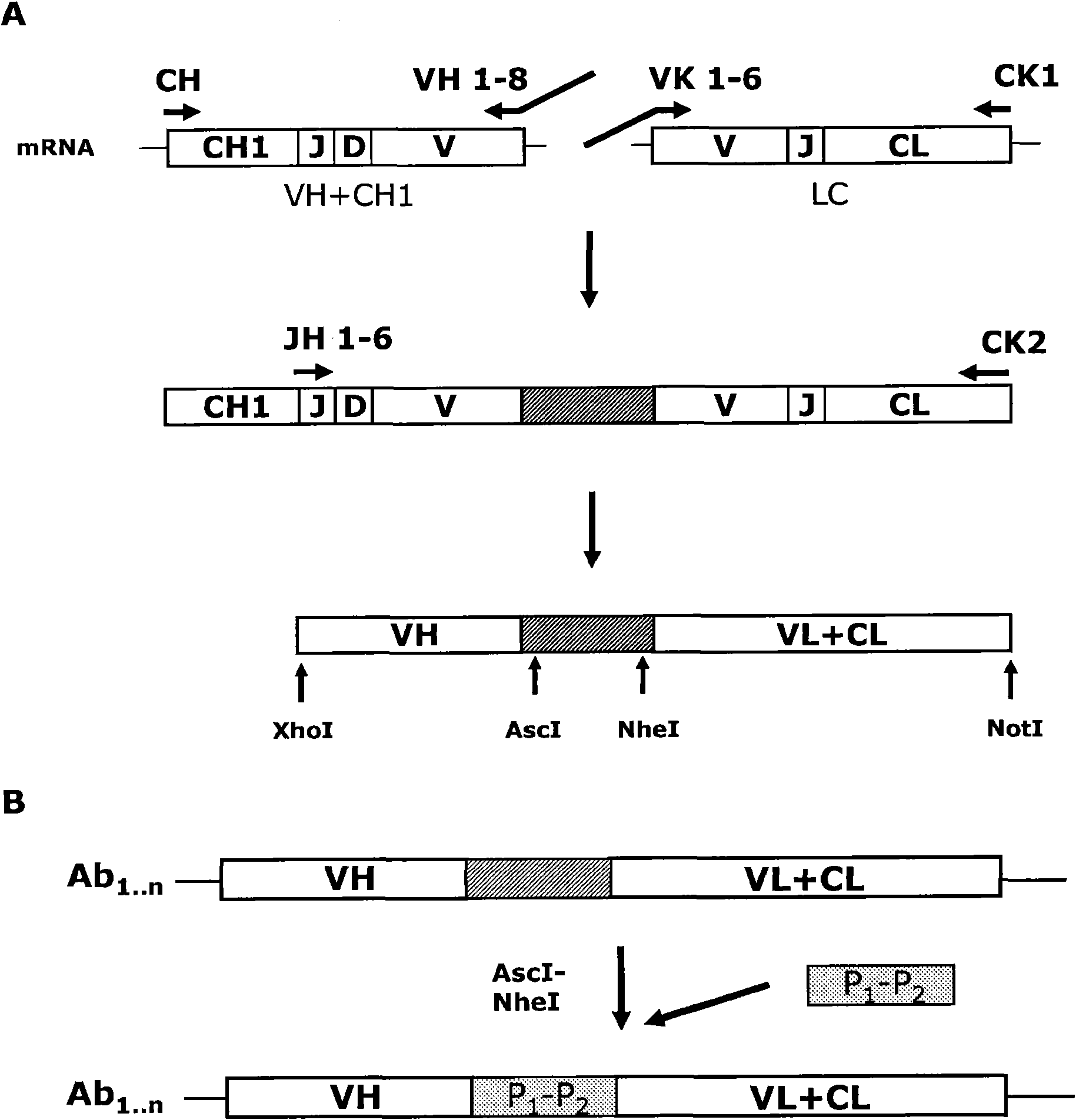
[0222] Peripheral blood mononuclear cells (PBMCs) were isolated from blood drawn from donors using Lymphoprep (Axis Shield) and gradient centrifugation according to the manufacturer's instructions. Isolated PBMC were either refrigerated at -150°C in FCS, 10% DMSO or used directly. The B cell fraction was labeled with an anti-CD19 antibody and isolated from the PBMC fraction using magnetic cell sorting (MACS). PBMC (1x10 6 cells) were incubated with anti-CD19-FITC-conjugated antibody (BD Pharmingen) at 4°C for 20 minutes. Cells were washed twice and resuspended in MACS buffer (Miltenyi Biotec). Anti-FITC microbeads (Miltenyi Biotec) were mixed with labeled cells and incubated at 4°C for 15 minutes. The washing procedure was repeated before applying the cell-bead suspension to a LS MACS column (Miltenyi Biotec). The ...
Embodiment 2
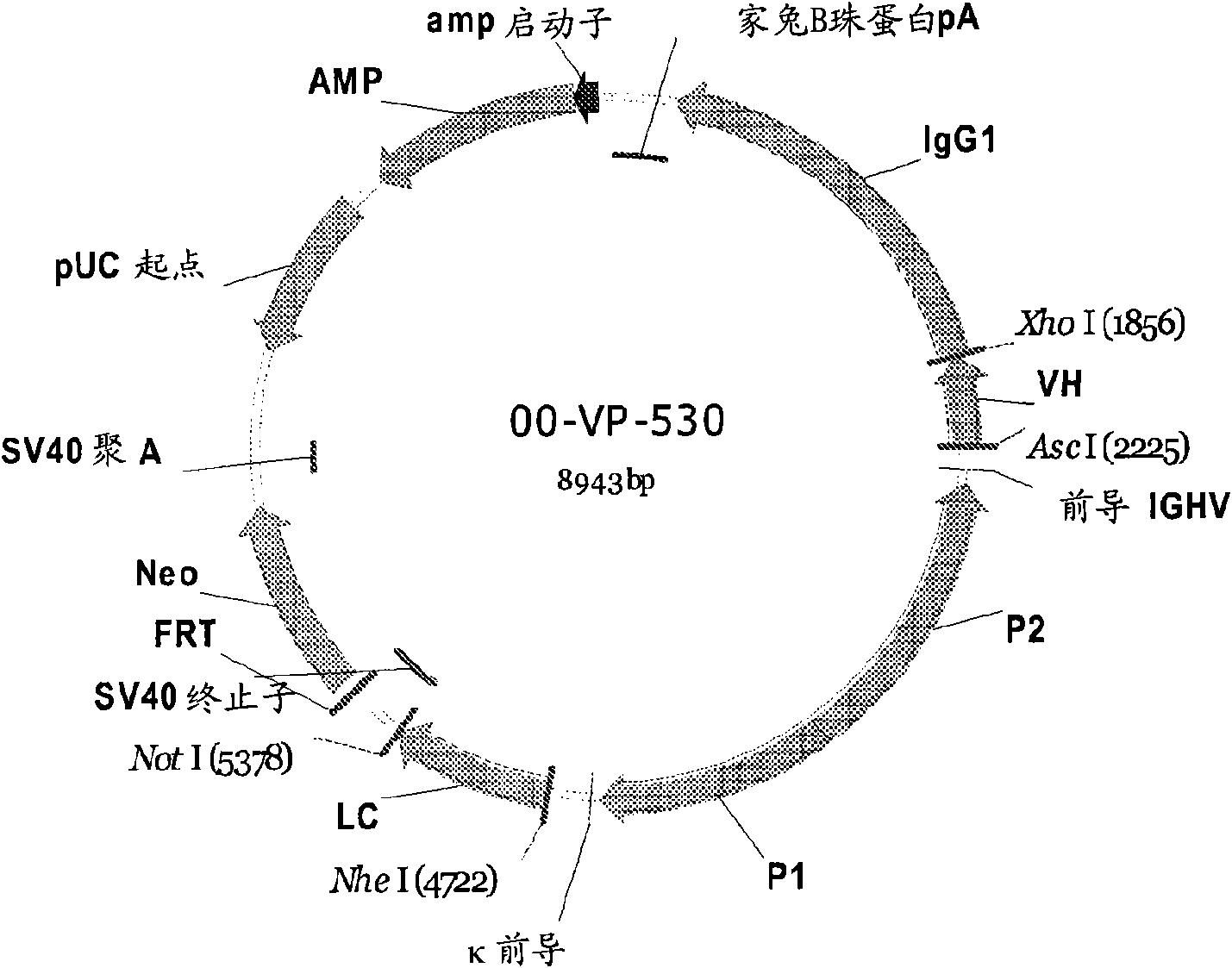
[0349] In this example, exemplifying the detection of a cognate V protein containing a full-length antibody expressed as having anti-RSV specificity. H and V L Isolation, screening, selection and banking of the right clones.
[0350] donor
[0351] A total of 89 donors were recruited from employees and parents of children who had been admitted to the pediatric department of Hvidovre Hospital (Denmark) during the RSV season. An initial blood sample of 18ml was drawn, for CD19 + B cells were purified (Example 1, part a), screened for the presence of anti-RSV antibodies using ELISpot (Example 1, part b), and the frequency of plasma cells was determined by FACS analysis.
[0352] Eleven donors were found to be positive in the initial blood sample screening, from 10 of which a second blood sample of 450ml was collected. Plasmablasts were subjected to single cell sorting according to part a of Example 1. ELISpot was performed on the fraction of CD19 positive B cells.
[0353] ...
Embodiment 3
[1573] In vitro neutralization experiments have been performed with single antibody clones and with combinations of purified antibodies. All antibody mixtures described below consist of a number of individual anti-RSV antibodies of the invention, which were combined into mixtures using equal amounts of different antibodies.
[1574] Single Antibody Test
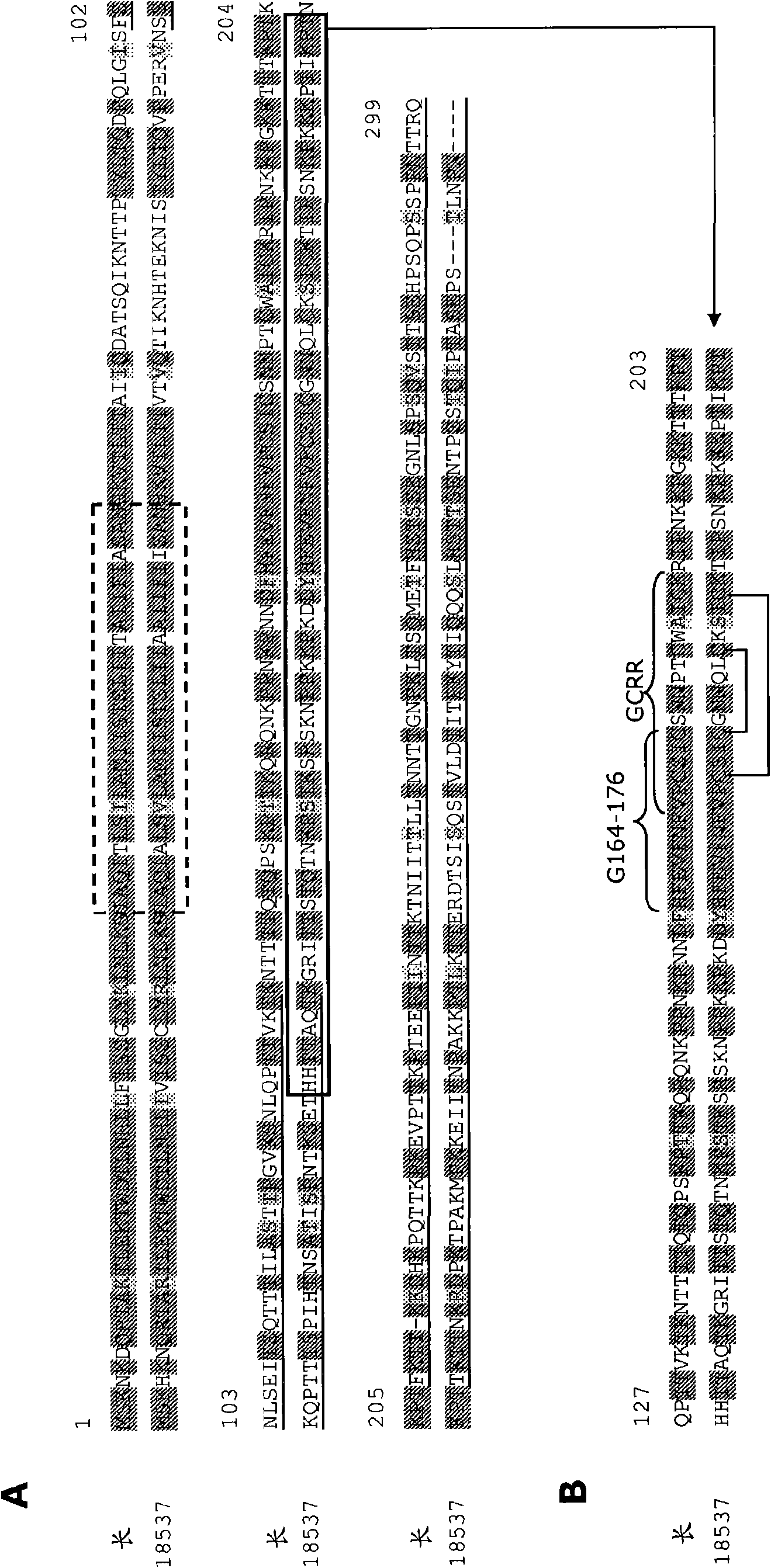
[1575] First, the neutralizing activity of each antibody was determined in PRNT in the presence of complement against RSV subtype A and B strains, as described above in Example 1, section j-2. The EC of a number of purified antibodies are shown in Table 8 50 value. Interestingly, although most anti-F antibodies individually exhibited virus neutralizing activity, EC could not be determined for most anti-RSV protein G antibodies. 50 value. This could be interpreted as indicating that these antibodies cannot neutralize the virus individually. However, subsequent refinement of the assay also yielded EC for G-responsive clone...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com