Deoxynivalenol immune affinity column and preparation method and use thereof
A technology of deoxynivalenol and immunophile, which is applied in the direction of chemical instruments and methods, separation methods, preparation of test samples, etc., can solve the problems affecting the efficiency of deoxynivalenol and the linearity of the standard curve. Good, uncontrollable directionality and other problems, to save time and cost, easy to operate, and improve purification efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Preparation of deoxynivalenol immunoaffinity purification column
Embodiment approach
[0044] A preferred embodiment of the present invention to prepare deoxynivalenol immunoaffinity purification column is as follows:
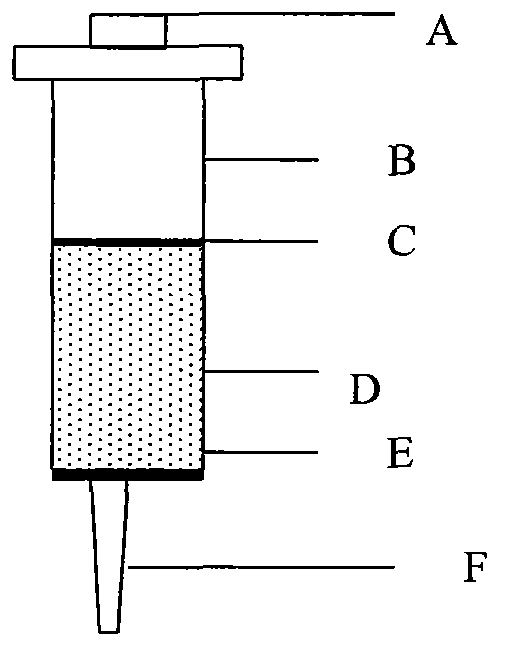
[0045] 1. Agarose Gel Activation
[0046] Take 2% agarose gel sepharose 2B and wash it with 20 times the volume of distilled water to wash away the remaining ethanol. Use a funnel to filter out the water. Weigh 5 grams of the wet gel after filtering off the water, add 7.5 milliliters of 0.8M NaOH, 2 milliliters of 30% epichlorohydrin, 2 milliliters of sodium borohydride NaBH4, 5 milliliters, shake the table at 25 ° C React for 8 hours.
[0047] After the reaction, wash with 50 ml of distilled water to remove the mixed epichlorohydrin in the gel.
[0048] 2. Coupling of Protein G to Activated Sepharose
[0049] Take 1 gram of activated agarose gel, and use coupling buffer (0.1M NaHCO 3 , 0.8M NaCl, pH8.9) and washed 3 times. Add 20ml of 2mg / ml protein G, and couple at room temperature for 4 hours.
[0050] The coupled agarose carrier was wa...
Embodiment 2
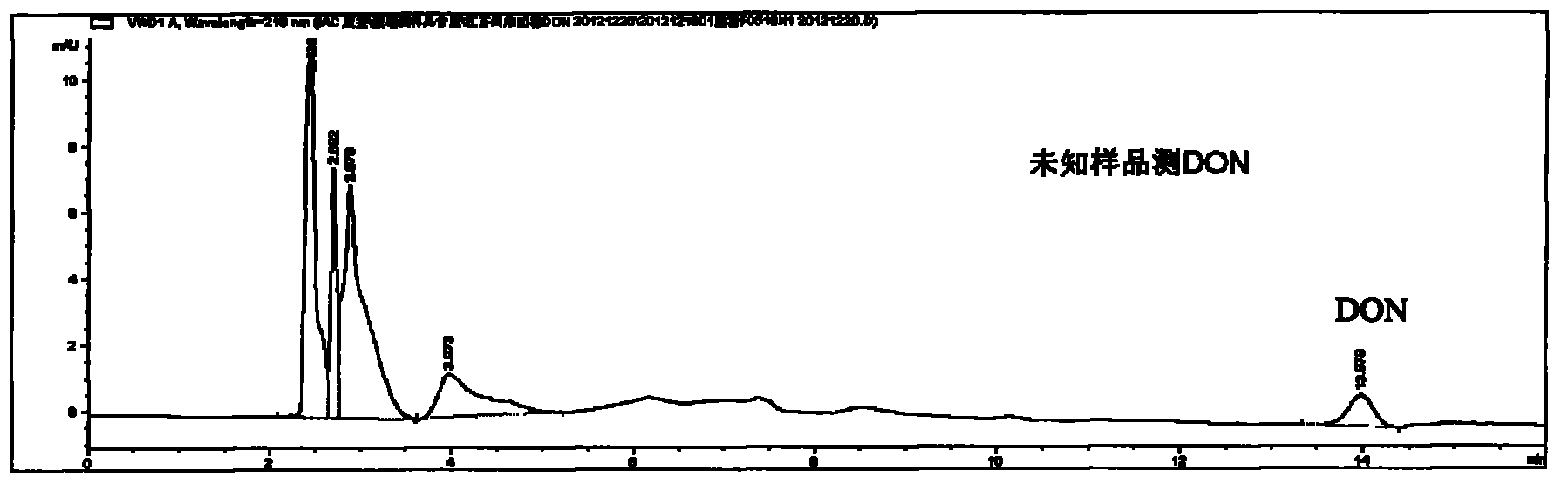
[0062] Example 2: Purification and detection of deoxynivalenol in flour using a deoxynivalenol immunoaffinity column
[0063] 1. Flour sample processing:
[0064] ----Weigh 25g of flour sample, 5g of polyethylene glycol, add 100ml of distilled water;
[0065] ---- Vigorously oscillate on the shaker for 20 minutes to make it completely mixed;
[0066] ----Filter with microfiber filter paper and collect the filtrate;
[0067] ----Take 1mL of filtrate for sample loading detection.
[0068] 2. Equilibrate the deoxynivalenol immunoaffinity purification column at room temperature for 30 minutes.
[0069] 3. Take out the immunoaffinity column, connect the injection port with the syringe barrel, and connect the syringe to the air-controlled operating frame.
[0070] 4. Wash the affinity column 3 times with deionized water, 10 ml each time, and adjust the air pump pressure of the stomata operation frame to make the liquid
[0071] The body flows out at a flow rate of 3 drops / secon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com