G-eGFP protein, preparation method, and application
A fusion protein and nucleotide sequence technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, applications, etc., can solve the problems of low toxicity of chemical fluorescein, complex coupling steps, instability, etc., and achieve broad The effect of applying the foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
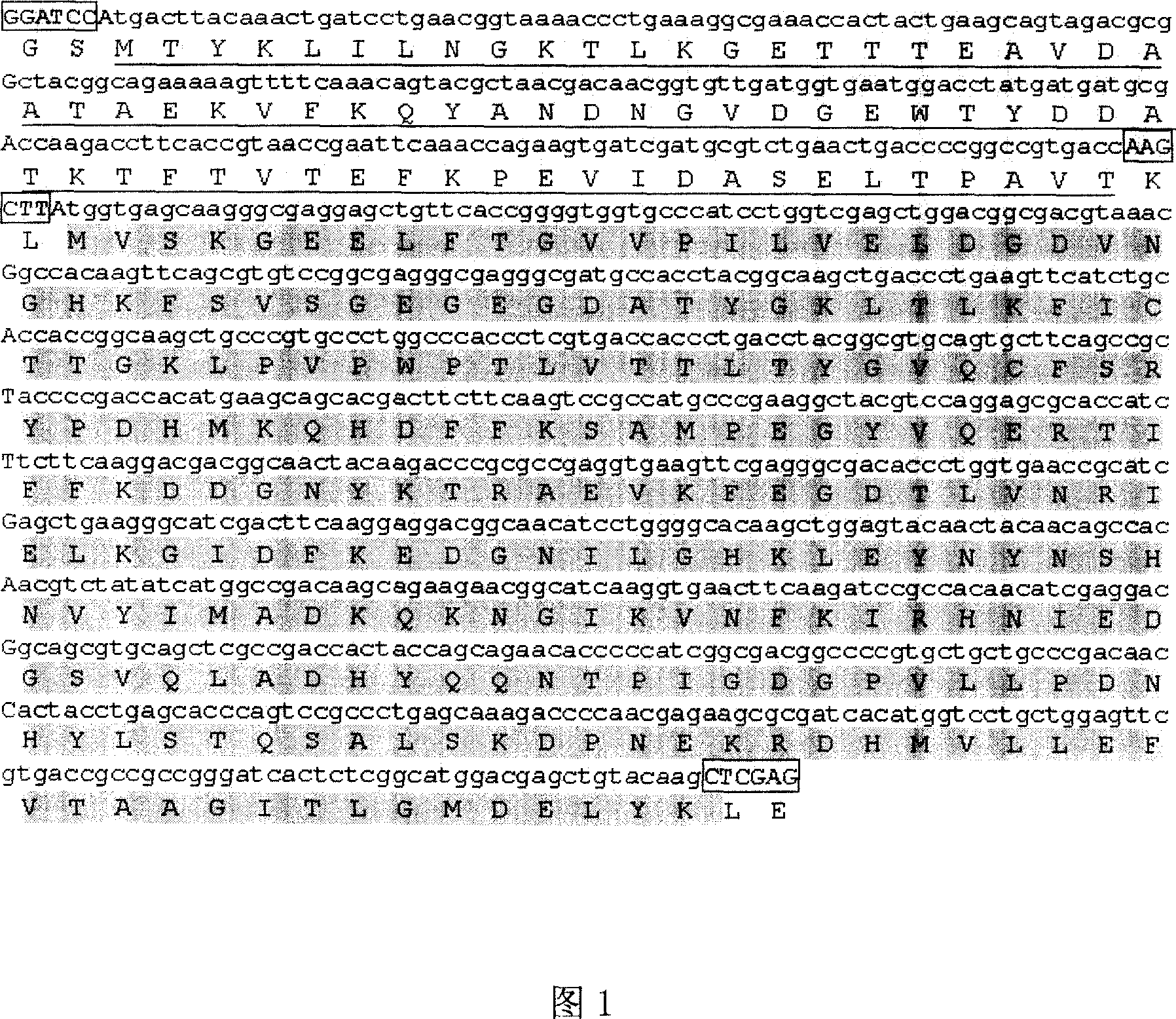
[0033] Synthesis of G-eGFP Gene Sequence
[0034] According to the amino acid sequence of the binding antibody region in Streptococcus sp.GX7805 protein G (data source: GenBank Y00428), codons were optimized and designed, and the designed nucleotide sequence was provided by Shanghai Sangon Bioengineering Co., Ltd. (see appendix Nucleotide sequence and amino acid sequence of Fig. 1G-eGFP fusion gene. Square box represents the restriction site of insertion, and underline represents ProteinG amino acid sequence, and gray represents eGFP amino acid sequence.) and Streptococcus Streptococcus protein G coding nucleotide sequence and The amino acid sequences are 85% identical and 15% different, with BamHI and HindIII restriction sites at both ends; the designed sequence is synthesized and then cloned into the pMD18-T vector. The coding region of eGFP gene was obtained by PCR amplification from pEGFP-N1 (Zhang Chuanxi, Sun Jianxin, Shi Huijuan, Chen Zheyu, Wu Xiangfu, "Highly High Expre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com