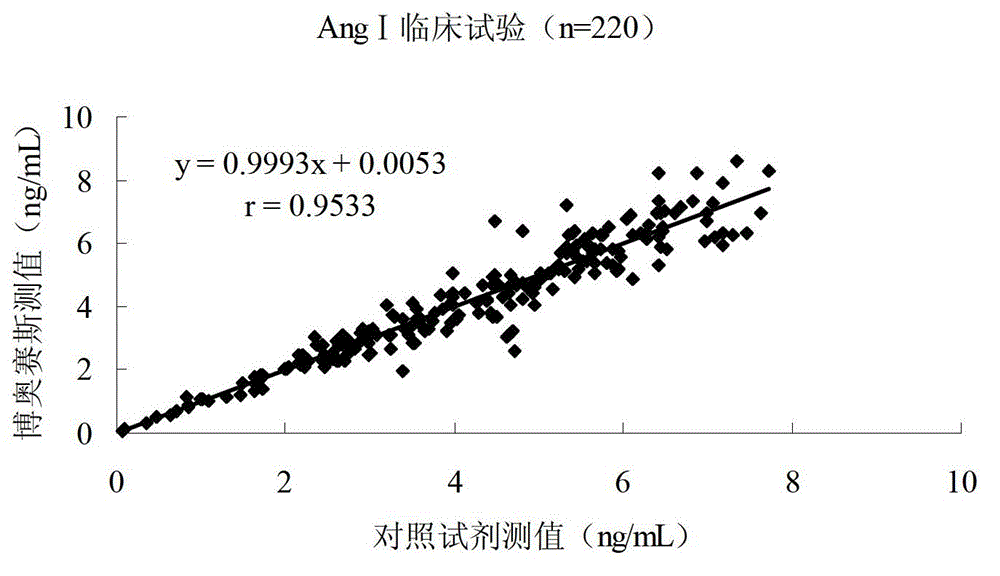
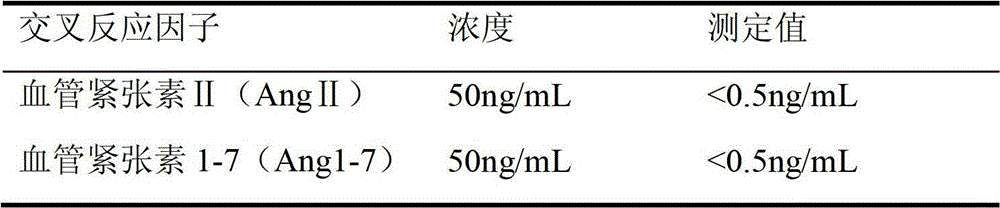
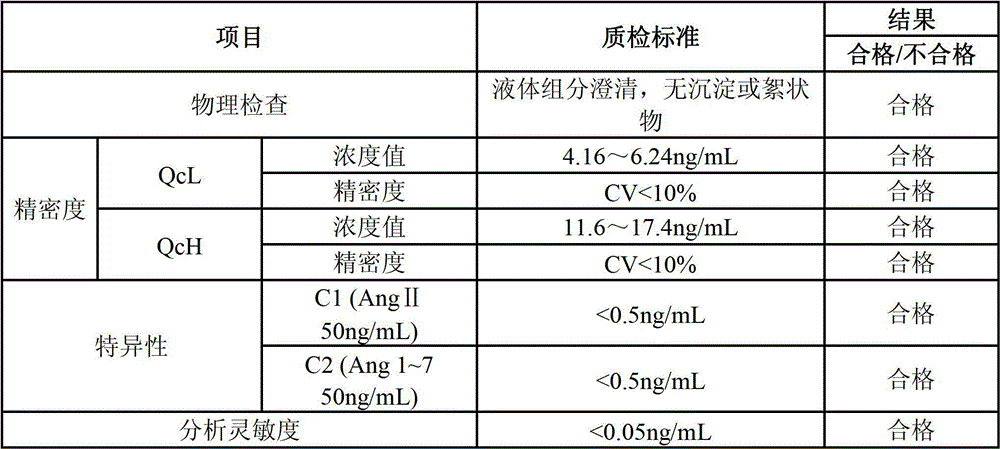
Quantitative detection kit combining magnetic particles with chemiluminescence immunoassay for angiotensin (Ang) I, and preparation method of kit
A chemiluminescence immunoassay and angiotensin technology, which is applied in the field of immunoassay medicine, can solve the problems of short validity period of reagents, narrow detection range, and low sensitivity, and achieve the effects of good stability, easy operation, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of Angiotensin Ⅰ (Ang Ⅰ) Magnetic Particle Chemiluminescent Immunoquantitative Assay Kit Ⅰ
[0032] (1) Preparation of Ang Ⅰ calibrator:
[0033] Dilute the Ang Ⅰ pure product with 50% bovine serum to form a series of gradients, the concentrations are 0, 0.8, 2, 10, 20, 50ng / mL;
[0034] (2) Preparation of Ang Ⅰ quality control substance:
[0035] The Ang I antigen was prepared with 50% bovine serum for low-value quality control and high-value quality control, and the concentrations were 4.16ng / mL and 17.4ng / mL;
[0036] (3) Preparation of magnetic particle-streptavidin suspension:
[0037]Take 100mL0.1mol / L 2-morpholineethanesulfonic acid solution (MES solution), add 10mg magnetic particles and 3mg streptavidin (SA) in turn, stir for 30min, then add 10mg / mL carbodiimide hydrochloride Solution (1-ethyl-(3-dimethylaminopropyl, EDC) 3.5uL, after reacting for 1h, use a magnetic stand to absorb, stand still for 10min, remove the liquid, add 10mL 0....
Embodiment 2
[0059] Example 2: Preparation of Angiotensin Ⅰ (Ang Ⅰ) Magnetic Particle Chemiluminescent Immunoquantitative Assay Kit Ⅱ
[0060] (1) Preparation of Ang Ⅰ calibrator:
[0061] Dilute the Ang Ⅰ pure product with 50% bovine serum to form a series of gradients, the concentrations are 0, 0.8, 2, 10, 20, 50ng / mL;
[0062] (2) Preparation of Ang Ⅰ quality control substance:
[0063] The Ang I antigen was prepared with 50% bovine serum for low-value quality control and high-value quality control, and the concentrations were 6.24ng / mL and 11.6ng / mL;
[0064] (3) Preparation of magnetic particle-streptavidin suspension:
[0065] Take 100mL0.1mol / L 2-morpholineethanesulfonic acid solution (MES solution), add 10mg magnetic particles and 3mg streptavidin (SA) in turn, stir for 30min, then add 10mg / mL carbodiimide hydrochloride Solution (1-ethyl-(3-dimethylaminopropyl, EDC) 3.5uL, after reacting for 1h, use a magnetic stand to absorb, stand still for 10min, remove the liquid, add 10mL 0...
Embodiment 3
[0076] Example 3: Preparation of Angiotensin Ⅰ (Ang Ⅰ) Magnetic Particle Chemiluminescent Immunoquantitative Assay Kit III
[0077] (1) Preparation of Ang Ⅰ calibrator:
[0078] Dilute the Ang Ⅰ pure product with 50% bovine serum to form a series of gradients, the concentrations are 0, 0.8, 2, 10, 20, 50ng / mL;
[0079] (2) Preparation of Ang Ⅰ quality control substance:
[0080] Use Ang Ⅰ antigen containing 50% bovine serum to prepare low-value quality control and high-value quality control, the concentrations of which are 5.2ng / mL and 13.8ng / mL;
[0081] (3) Preparation of magnetic particle-streptavidin suspension:
[0082] Take 100mL0.1mol / L 2-morpholineethanesulfonic acid solution (MES solution), add 10mg magnetic particles and 3mg streptavidin (SA) in turn, stir for 30min, then add 10mg / mL carbodiimide hydrochloride Solution (1-ethyl-(3-dimethylaminopropyl, EDC) 3.5uL, after reacting for 1h, use a magnetic stand to absorb, stand still for 10min, remove the liquid, add 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com