Feline herpes virus type I gB-gD recombinant protein and preparation method and application thereof
A feline herpes virus and recombinant protein technology, applied in biochemical equipment and methods, viruses, viral peptides, etc., can solve problems such as difficult promotion and time-consuming, and achieve the effects of improving sensitivity, reducing cross-reaction, and pollution-free training
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The construction of embodiment 1 feline herpesvirus type I gB-gD fusion protein gene expression vector
[0064] Feline herpesvirus type I gB gene is designed according to the protein sequence of NCBI Gene bank: YP_003331552.2. According to the hydrophilicity and hydrophobicity of the protein ( https: / / web.expasy.org / protscale / ) analysis, the gB (1-100aa) sequence was selected for fusion in the area predicted to have a higher hydrophilic content. The gD gene was designed according to the protein sequence of NCBI Gene bank: YP_003331589.1. According to the hydrophilicity and hydrophobicity of the protein ( https: / / web.expasy.org / protscale / ) analysis, the gD (275-374aa) sequence was selected for fusion in the predicted higher hydrophilic content region.
[0065] The amino acid sequence of the gB-gD recombinant protein fusion protein is as follows (SEQ ID NO.1):
[0066] MSTRGDLGKRRRGSRWQGHSGYFRQRCFFPSLLGIAATGSRHGNGSSGLTRLARYVSFIWIVLFLVGPRPVEGQSGSTSEQPRRTVATPEVGG...
Embodiment 2
[0071] Embodiment 2 Expression of feline herpesvirus type I gB-gD fusion protein
[0072] Feline herpesvirus type I gB-gD fusion gene plasmid was transformed into Escherichia coli BL21, spread on LB plates containing 50 μg / mL kanamycin (Shanghai Sangong, product number: K0408), cultured overnight at 37°C, and picked Take a single clone colony, culture it with 300mL LB medium containing the same concentration of kanamycin at 37°C until the OD600 reaches about 0.6, and induce expression with IPTG (Shanghai Shenggong, product number: IB0168) with a final concentration of 1mM. For: 25 ℃, rotating speed 200rpm, 4h. After induction, the culture solution was centrifuged at 4° C. at 7000 rpm for 10 min to collect the bacteria.
Embodiment 3
[0073] Embodiment 3 Purification and renaturation of feline herpesvirus type I gB-gD fusion protein
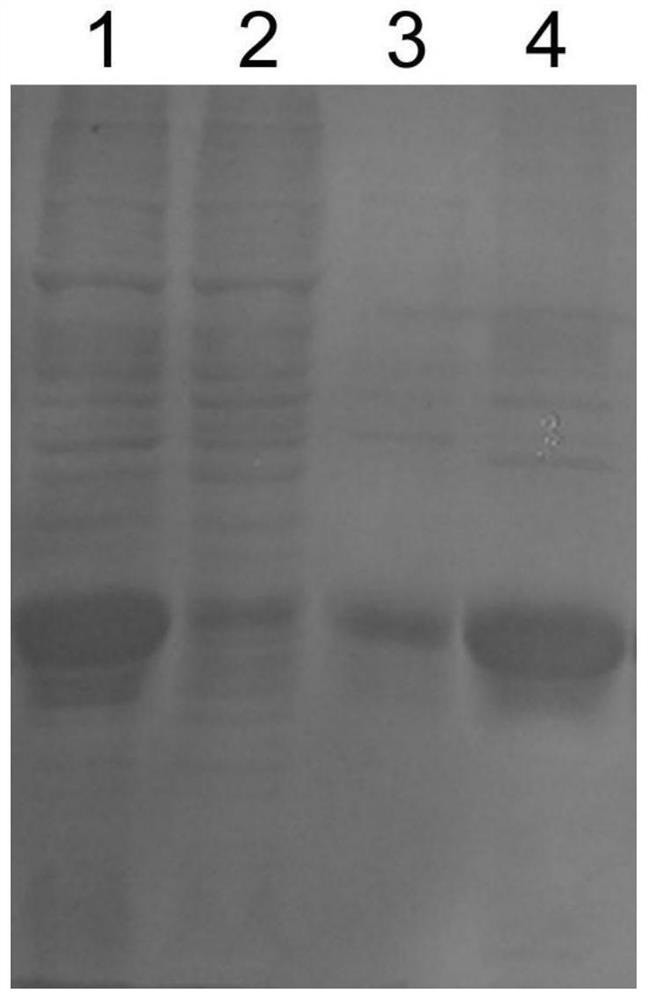
[0074] Use 50mL loading buffer Binding Buffer (50mM Tris, 0.2M Nacl, pH8.0) 50mL to crush the bacteria; then ultrasonically break, the condition is 600w, ultrasonic 2s, interval 5s, a total of 100 times; finally 12000rpm, 30min, 4℃ Collect the supernatant by centrifugation, and the target protein is in the supernatant. Then, it was purified by Ni column, and the target protein was eluted with Elution Buffer (50mM Tris, 0.2M Nacl, 0.5M Imidazole, pH8.0). The target protein was detected by PAGE gel electrophoresis, and the results were as follows figure 1 shown.
[0075] Depend on figure 1 It can be seen that the purified fusion protein has a high purity. The purified recombinant protein was dialyzed with a dialysis buffer (50mM Tris, 0.2M Nacl, pH8.0), and the dialysis solution was changed every 12 hours for a total of 3 times. The dialyzed protein solution was taken out, f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com