Human SARS-CoV-2 monoclonal antibody and preparation method and application thereof
A monoclonal antibody, sars-cov-2 technology, applied in the direction of botanical equipment and methods, biochemical equipment and methods, applications, etc., to achieve the effect of good specificity and high affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of embodiment 1 human SARS-CoV Nucleocapsid recombinant protein
[0036] (1) Construction of human SARS-CoV Nucleocapsid prokaryotic expression vector
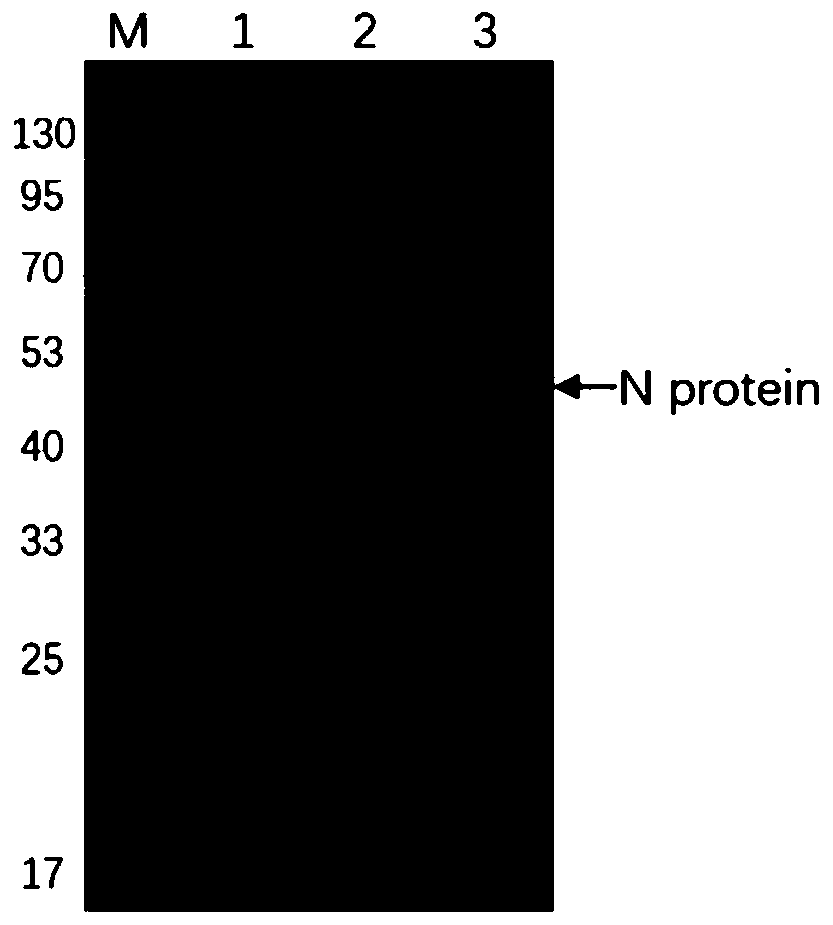
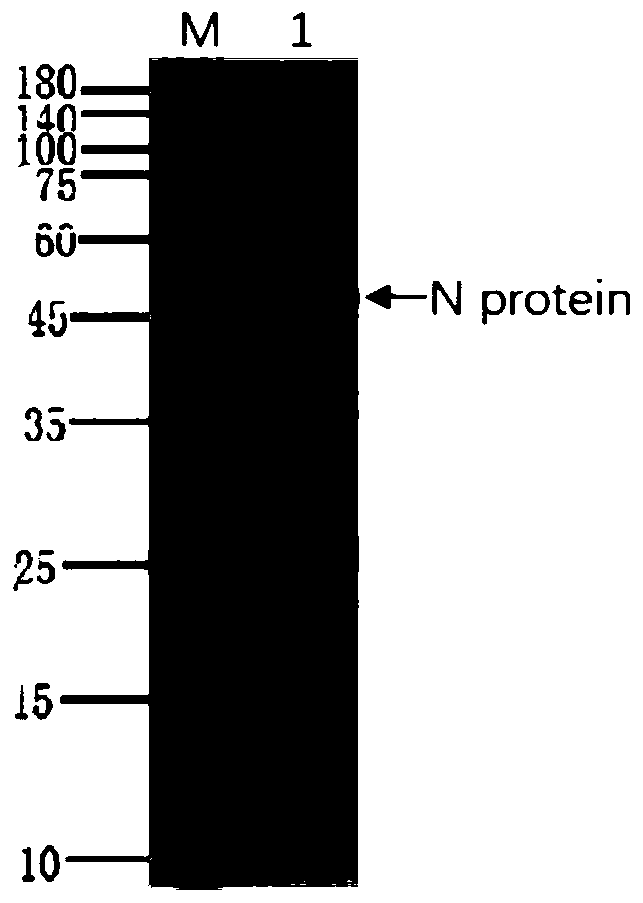
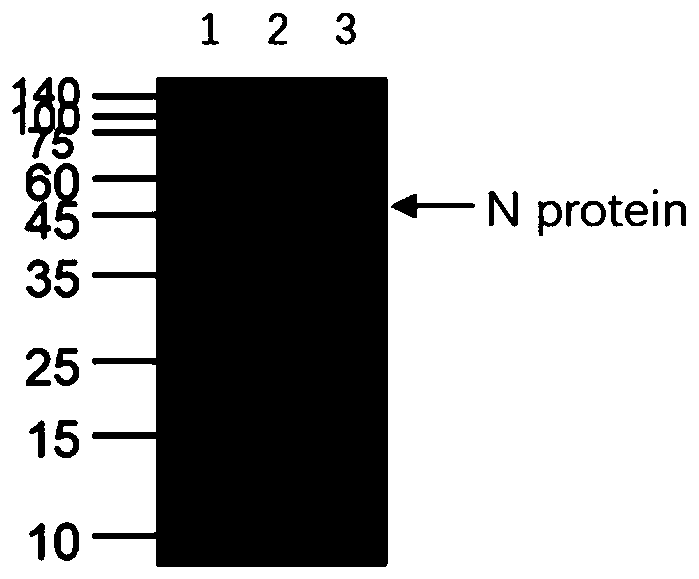
[0037] Human SARS-CoV Nucleocapsid gene sequence SEQ ID NO.2 was artificially synthesized, inserted into the prokaryotic expression plasmid pET2a digested with NdeI and XhoI, and transformed into competent Escherichia coli BL21(DE3). 0.1g / L kanamycin resistance plate screens positive clones, and induces them with a final concentration of 1mM IPTG at 16°C, 25°C, 30°C, and 37°C respectively, preferably at 25°C, shaking at 200rpm Cultured for 14 hours to induce human SARS-CoV Nucleocapsid recombinant protein expression. Finally identified by SDS-PAGE, the results are as follows figure 1 shown.
[0038] (2) Expression of pET28a-SARS-CoV-N fusion protein
[0039] The correctly identified pET28a-SARS-CoV-N strains were transferred to LB medium containing 100ug / ml kanamycin for overnight culture, and then t...
Embodiment 2
[0050] The preparation of embodiment 2 anti-human SARS-CoV-2 N monoclonal antibodies
[0051] (1) Animal immunity
[0052] Using purified human SARS-CoV-N recombinant protein as immunogen, immunize 8-12 week-old BALB / c female mice by conventional methods, emulsify with complete Freund's adjuvant and antigen for the first time, and immunize subcutaneously and intraperitoneally at multiple points. The dose is 100 μg / mouse; booster immunization once every two weeks, a total of three times, the adjuvant is Freund’s incomplete adjuvant, the dose is 100 μg / mouse / time; the tail blood titer of mice is detected by indirect ELISA method>1:10 4 , Three days before the fusion, intraperitoneal injection of 100 μg of human SARS-CoV-2 N antigen was carried out.
[0053] (2) cell fusion
[0054] Mix immune spleen cells and myeloma cells (SP2 / 0) at a ratio of 5:1, centrifuge at 1000rpm for 10min, discard the supernatant; resuspend the cells in RPMI-1640 medium, centrifuge at 1000rpm for 10min,...
Embodiment 3
[0070] Monoclonal antibody purification of embodiment 3 anti-human SARS-CoV-2 N recombinant protein
[0071] (1) Purification of monoclonal antibodies
[0072] The monoclonal antibody ascites obtained in Example 2 was purified by Protein A affinity chromatography, and the specific steps were as follows:
[0073] The basic steps are: equilibration-loading-equilibrium-elution-equilibrium-preservation.
[0074] Equilibrium solution: 20mM PB (pH7.2) buffer;
[0075] Eluent: 0.1M pH 3.0 glycine-HCl buffer;
[0076] Neutralization buffer: 1M, pH9.0, Tris-HCl buffer;
[0077] Preservation solution: 20% absolute ethanol.
[0078] Take 3-5ml of ascitic fluid, precipitate with 50% saturated ammonium sulfate for more than 2 hours, centrifuge at 10,000rmp for 20 minutes, discard the supernatant; dissolve the precipitate with equilibrium solution, filter it with a 0.45μm filter membrane, and prepare for loading; Protein A affinity column Pre-equilibrated with 5 times the column volume...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com