Chinese herbal composition for curing HIV and preparation method thereof
A composition and AIDS technology, which can be used in pharmaceutical formulations, active ingredients of hydroxyl compounds, and medical preparations containing active ingredients, etc., can solve the problems of drug resistance in patients, many side effects of therapy, and limited clinical application, and achieve non-toxicity. Effects of side effects, improved quality of life, low cost of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
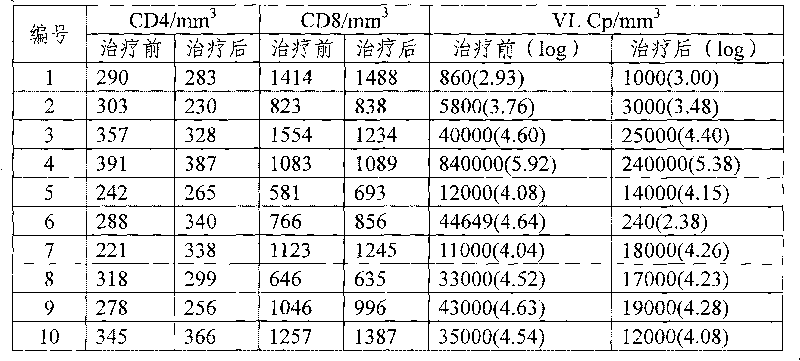
Examples
Embodiment 1
[0026] The preparation of the capsule of embodiment 1 Chinese medicine composition of the present invention
[0027] 1) Take 338g of ginseng and 255g of Panax notoginseng, add 70% ethanol equivalent to 8 times the amount of crude drugs, heat and reflux 3 times, each time for 2 hours, filter the medicinal residues, combine the extracts, concentrate under reduced pressure until the relative density is 1.13~ 1.15 (50°C) first thick paste;
[0028] 2) Mix the medicinal dregs with 170g of Tianhuafen, 170g of Qingdai, 170g of Zihuadiding, 170g of Dilong, and 170g of Radix Glycyrrhizae, add water equivalent to 8 times the amount of crude medicine, heat and reflux 3 times, each time for 2 hours, filter, Combine the extracts, concentrate under reduced pressure to the second thick paste with a relative density of 1.2 (50°C), add 95% ethanol to make the alcohol content reach 70%, leave it for 12 hours, filter, and concentrate under reduced pressure to a relative density of 1.27-1.29 (50...
Embodiment 2
[0032] The preparation of the tablet of embodiment 2 Chinese medicine composition of the present invention
[0033] 1) Take 300g of ginseng, 200g of Panax notoginseng, and 120g of Qingdai, add 60% ethanol equivalent to 6 times the amount of crude drugs, heat and reflux twice, each time for 1 hour, filter the medicinal residues, combine the extracts, and concentrate under reduced pressure to relative density The first thick paste of 1.10~1.12(50℃);
[0034] 2) Mix the medicinal dregs with 120g of trichosanthemi, 120g of zihuafen, 120g of earthworm, and 120g of licorice, add water equivalent to 6 times the amount of crude drug, heat and reflux twice, each time for 1 hour, filter, and combine the extracts , concentrated under reduced pressure to the second thick paste with a relative density of 1.10 to 1.12 (50°C), added 95% ethanol to make the alcohol content reach 60%, left it for 10 hours, filtered, concentrated under reduced pressure to a relative density of 1.20 to 1.24 ( 5...
Embodiment 3
[0038] Embodiment 3 The preparation of the granule of Chinese medicine composition of the present invention
[0039] 1) Take 400g of ginseng, 300g of Panax notoginseng, and 220g of Qingdai, add 80% ethanol equivalent to 12 times the amount of crude drugs, heat and reflux 3 times, 4 hours each time, filter the medicinal residues, combine the extracts, and concentrate under reduced pressure to relative density The first thick paste of 1.20~1.25(50℃);
[0040] 2) Mix the medicinal dregs with 220g of trichosanthemi, 220g of zihuafen, 220g of earthworm, and 220g of licorice, add water equivalent to 12 times the amount of crude drug, heat and reflux 3 times, each time for 4 hours, filter, and combine the extracts , concentrated under reduced pressure to the second thick paste with a relative density of 1.20~1.25 (50°C), added 95% ethanol to make the alcohol content reach 80%, left it for 24 hours, filtered, concentrated under reduced pressure to a relative density of 1.35~1.40 ( 50...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com