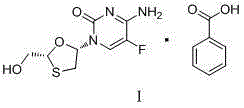
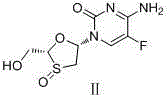
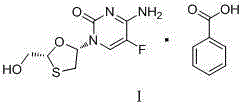
Emtricitabine benzoate, preparation method thereof, and method of preparing emtricitabine from emtricitabine benzoate
A technology of emtricitabine and benzoic acid, applied in the field of biochemistry, can solve the problems of poor product quality of emtricitabine, poor solid form of emtricitabine salt, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the preparation of emtricitabine benzoate
[0023] Add 160g of dipotassium hydrogen phosphate and 180g of water into the reaction flask equipped with a stirrer and a thermometer, stir until the dipotassium hydrogen phosphate is dissolved, add 120g of FCME and 1L of ethanol, and drop the mixed solution (2.4ml25% Aqueous sodium hydroxide solution, 48.6g potassium borohydride, 240ml water). After dropping, stir at 15-30°C for 2 hours. Use hydrochloric acid to adjust the pH to 4.0-4.5, and then use sodium hydroxide aqueous solution to adjust the pH to 6.8-7.2. Remove most of the ethanol under reduced pressure, and wash the remaining liquid with toluene three times. Move the water phase into a 1L reaction flask, add 36.7g of benzoic acid after heating to 40°C, keep stirring at 40-50°C for 2 hours, filter, wash with water, and dry the filter cake to obtain 95g of emtricitabine benzoate, The yield is 85%. MP: 121.2-122.6°C, 1 HNMR (DMSO) 400MHzδ8.211~8.228 (d...
Embodiment 2
[0024] Embodiment 2. Preparation of Emtricitabine Benzoate
[0025] Add 160g of dipotassium hydrogen phosphate and 180g of water into the reaction flask, stir until the dipotassium hydrogen phosphate dissolves, add 120g of FCME and 1L of ethanol, and drop the mixed solution (2.4ml of 25% sodium hydroxide aqueous solution, 34.13g of boron sodium hydride, 240ml water). After dropping, stir at 15-30°C for 5 hours. Use hydrochloric acid to adjust the pH to 4.0-4.5, and then use sodium hydroxide aqueous solution to adjust the pH to 6.8-7.2. Remove most of the ethanol under reduced pressure, and wash the remaining liquid with toluene three times. Move the water phase into a 1L reaction flask, add 36.7g of benzoic acid after heating to 40°C, keep stirring at 40-50°C for 2 hours, filter, wash with water, and dry the filter cake to obtain 85g of emtricitabine benzoate. Yield 77%.
Embodiment 3
[0026] Embodiment 3. Preparation of Emtricitabine
[0027] Add 95g of emtricitabine benzoate and 475g of isopropyl acetate into a 1L reaction flask, add 40.8g of triethylamine dropwise under stirring, after the drop is complete, keep stirring at 15-30°C for 4 hours, filter with suction, and filter the cake Wash twice with isopropyl acetate. The filter cake was dried to obtain 57.2 g of white solid emtricitabine. Yield 90%, purity greater than 99%, MP: 152.8-153.5°C.
[0028] 1 HNMR (DMSO) 400MHzδ8.18~8.20(d,J=8.0,1H),7.56,7.80(weak,2H),6.13~6.16(m,1H),5.38~5.41(weak,1H),5.18~5.20( t, J=4.0,1H), 3.82~3.71(2m,2H), 3.40~3.45(dd, J=12.0,1H), 3.11~3.15(dd,J=12.0,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com