Suitqable to industrialized method for preparing emtricitabine
A technology of emtricitabine and catalyst, applied in the field of preparation of emtricitabine, can solve problems such as being unsuitable for industrialized large-scale production, loss of intermediates, inability to reproduce, etc., and achieves easy large-scale production, low cost and high safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
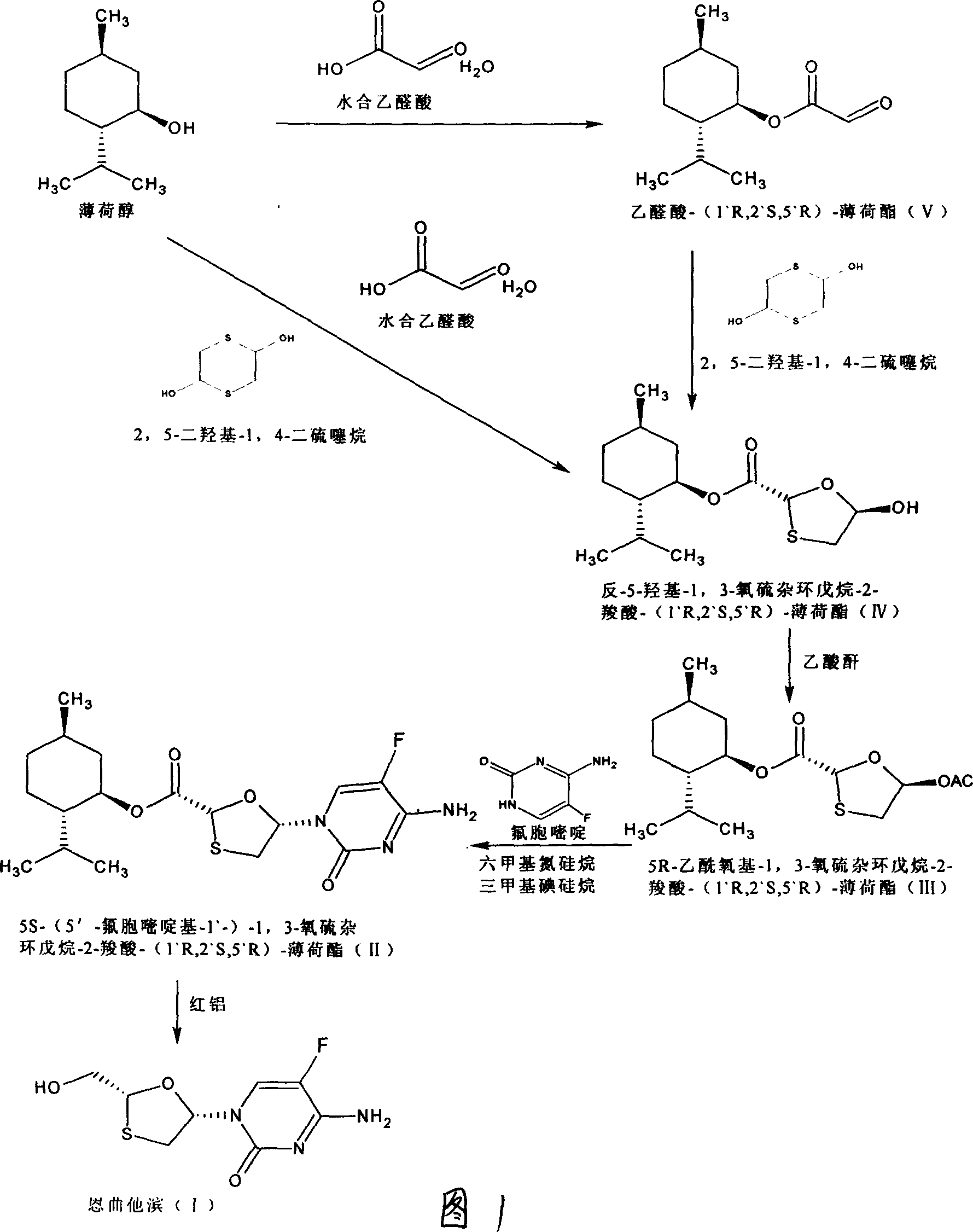
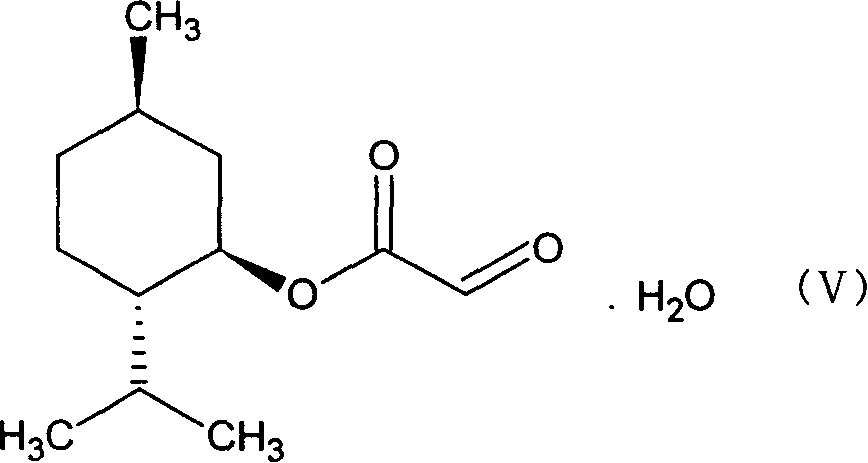
[0024] (1) Preparation of glyoxylic acid-(1`R, 2`S, 5`R)-menthyl ester (V)
[0025]
[0026] In a three-necked round flask equipped with a water separator, put 0.11mol of solid glyoxylic acid at room temperature, add 120ml of methyl ether, 0.1mol of menthol, and 1.5g of p-toluenesulfonic acid, heat and stir to dissolve the solid completely, and heat to reflux for 7- After 8 hours, the reaction solution was cooled to room temperature, filtered, washed with 50ml*3 water, the organic phase was collected, dried with anhydrous sodium sulfate overnight, filtered, the solvent was removed under low pressure, the remaining solid was dissolved with a small amount of petroleum ether, and a white solid was obtained 22.4g, melting range: 77-82°C, yield: 90%.
[0027] (2) Preparation of (2R, 5R)-5-hydroxyl-1,3-oxathiolane-2 carboxylic acid-(1`R, 2`S, 5`R)-menthyl ester (IV)
[0028]
[0029] Put 0.1mol glyoxylic acid-(1`R,2`S,5`R)-menthyl ester, 120ml methyl ether, 2,5-dihydroxy-1,4 ...
Embodiment 2
[0040] Preparation of (2R,5R)-5-hydroxy-1,3-oxathiolane-2-carboxylic acid-(1`R,2`S.5`R)-menthyl ester (IV)
[0041]
[0042] In a three-necked round flask equipped with a water separator, put 0.11mol of solid glyoxylic acid at room temperature, add 120ml of methyl ether, 0.1mol of menthol, and 1.5g of p-toluenesulfonic acid, heat and stir to dissolve the solid completely, and heat to reflux for 7- After 8 hours, cool the reaction solution to room temperature, filter, wash with 50ml*3 water, collect the organic phase, transfer it to a three-necked circular flask, add 0.05mol of 5-dihydroxy-1,4-dithiothiane, heat and stir to 40 ℃, until the white solid is completely dissolved, heat and reflux for 5-6 hours, cool the reaction solution to room temperature, filter, remove the solvent under low pressure, dissolve the remaining white solid with a small amount of petroleum ether, and freeze to obtain an odorous white solid. Yield 45 %, melting range: 110-112°C.
Embodiment 3
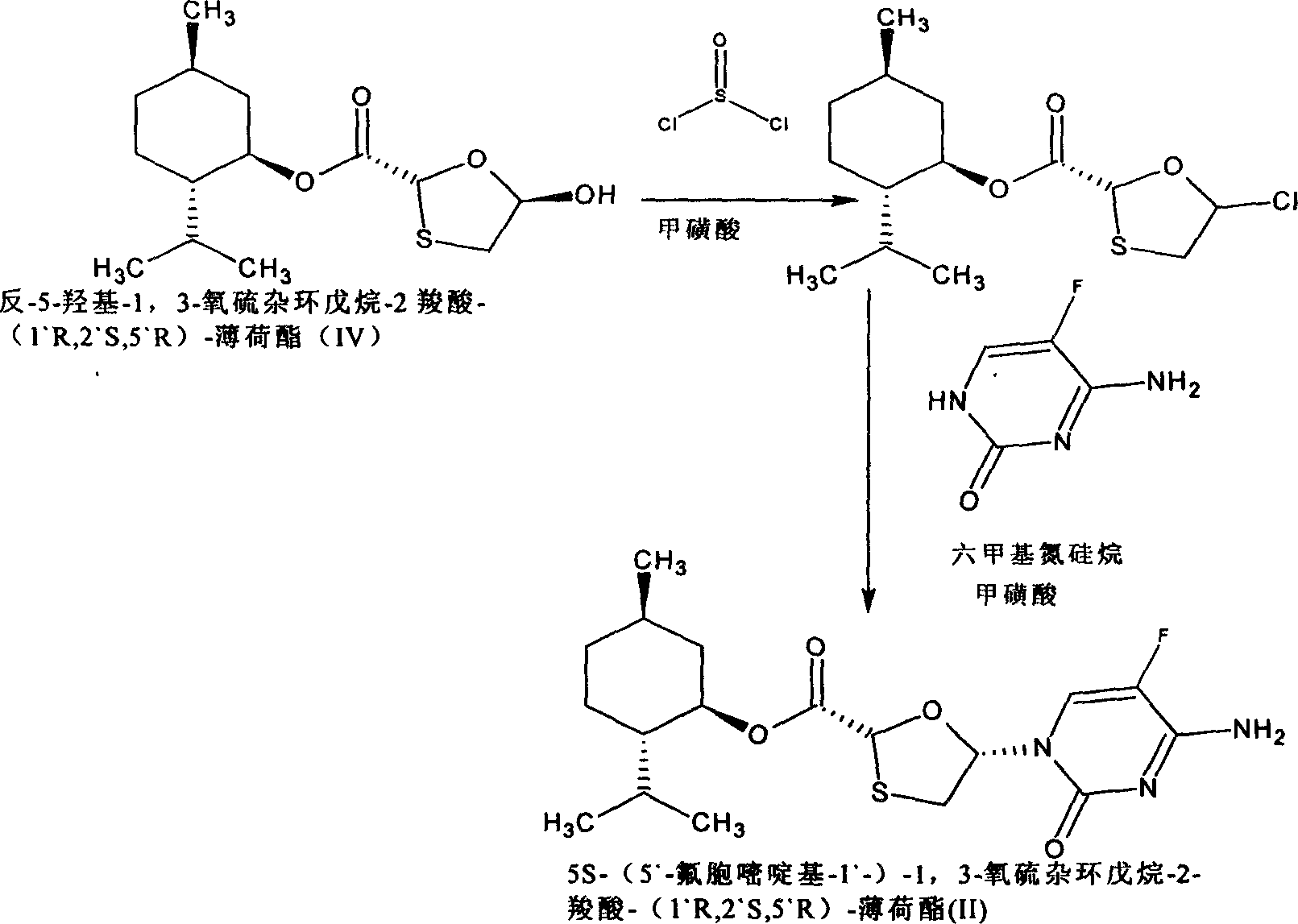
[0044] 5S-(5`-fluorocytosine-1`-yl)-1,3-oxathiolane-2-carboxylic acid-(1`R, 2`S, 5`R)-menthyl ester (II ) preparation
[0045]
[0046] (1) Add 3.0g (2R, 5R)-5-hydroxyl-1,3-oxathiolane-2-carboxylic acid-(1`R, 2`S, 5`R) into a round bottom flask - Menthyl ester, dissolved in 30ml of dichloromethane, add dropwise 1ml of N,N-dimethylformamide solution dissolved with two drops of methanesulfonic acid, cool to about 8°C, slowly drop into 0.8ml of thionyl chloride, The mixture was stirred at 10-15°C for 1.5 hours, evaporated to remove about 20ml of solvent under normal pressure, cooled to room temperature, and set aside.
[0047] (2) Add 1.34 grams of 5-fluorocytosine, 3 ml of hexamethylsilazane, 3 ml of toluene, and two drops of methanesulfonic acid in a round-bottomed flask, heat to reflux, and reflux for 1.5 hours to form a colorless solution. In some cases, add 1.5ml of triethylamine dropwise, then slowly drop into the solution obtained in (1), wash and add with 3ml of dich...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com