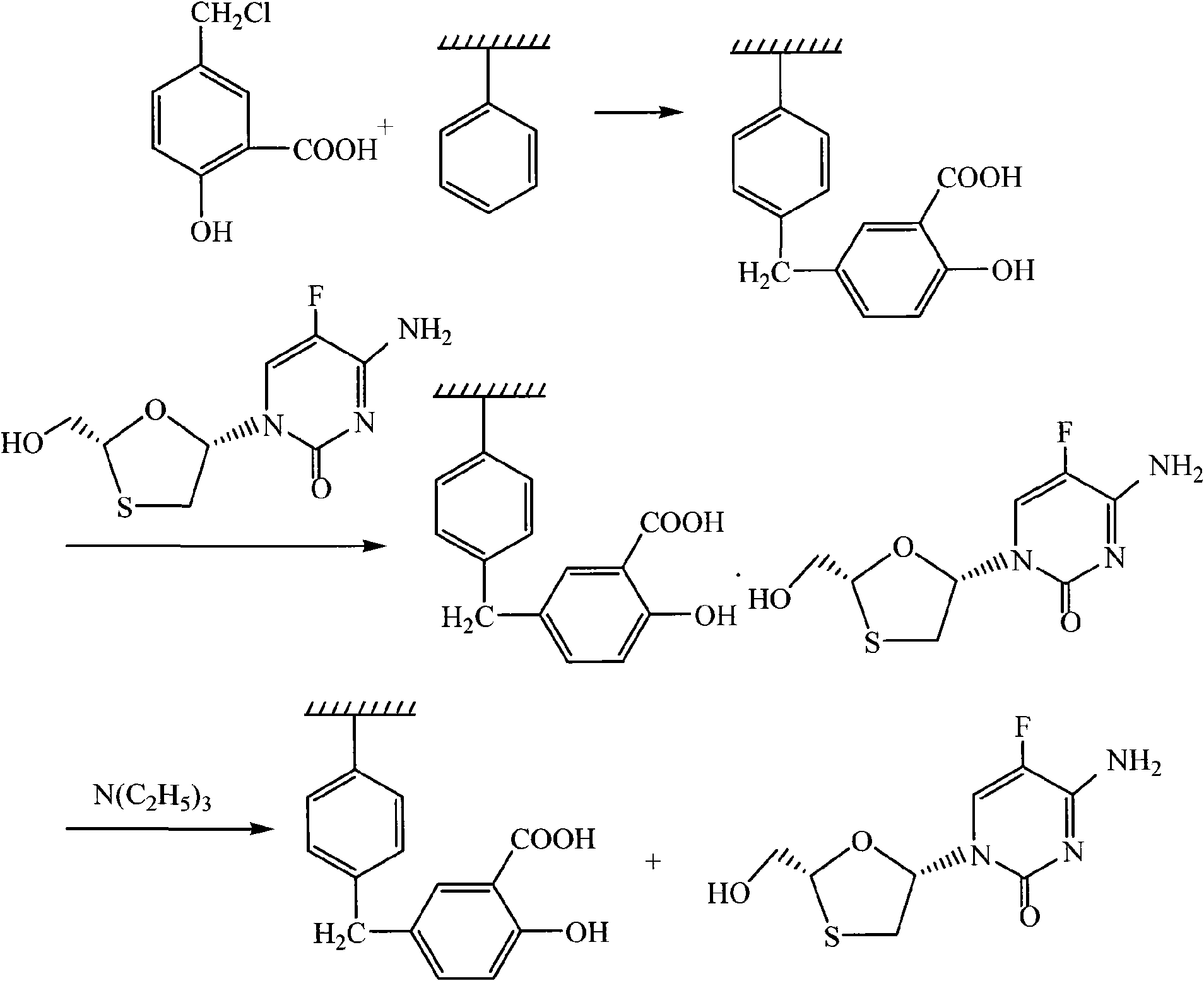
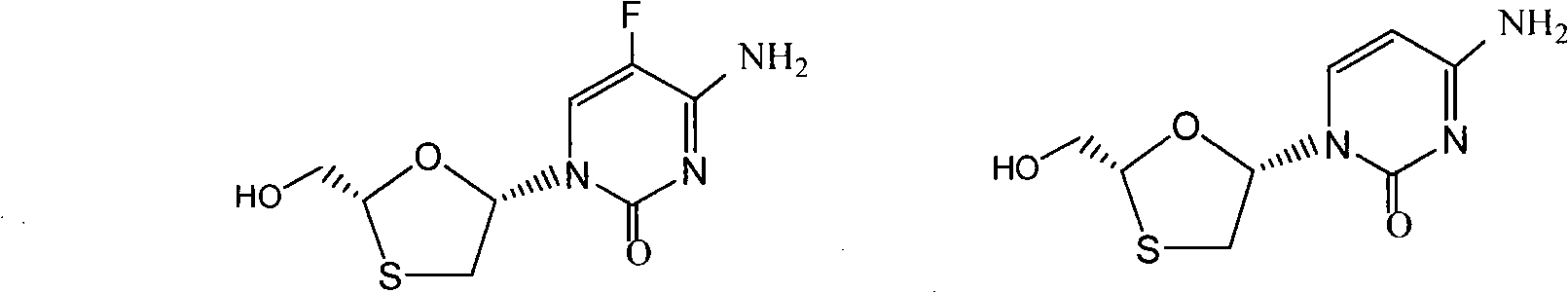
Method for separating emtricitabine
A technology of emtricitabine and separation method, which is applied in the field of chemical processing, can solve problems such as poor product quality and salicylic acid residue, and achieve the effects of simple technology, reduced pollution, and safe and reliable operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Concentrate the reaction filtrate containing about 4g of emtricitabine to dryness, dissolve it in 30ml of methanol, add 20g of resin, add 210ml of dichloromethane, and stir for 15min; heat to reflux for 7h, cool to room temperature, filter, wash with methanol, and dry to constant weight. Pour the dried polymer resin into a round bottom flask, add 30ml of methanol, 210ml of dichloromethane, and 2ml of triethylamine, heat to reflux for 7h, filter, wash with methanol, evaporate the filtrate to dryness, and weigh 2.54g. Yield 63.5%.
Embodiment 2
[0027] Concentrate the reaction filtrate containing about 4 g of emtricitabine to dryness, dissolve it in 250 ml of methanol, add 20 g of resin, and stir for 15 min; heat to reflux for 7 h, cool to room temperature, filter, wash with methanol, and dry to constant weight. Pour the dried polymer resin into a round bottom flask, add 30ml of methanol, 210ml of dichloromethane, and 2ml of triethylamine, heat to reflux for 7h, filter, wash with methanol, evaporate the filtrate to dryness, and weigh 1.73g. Yield 43.2%.
Embodiment 3
[0029] Concentrate the reaction filtrate containing about 4 g of emtricitabine to dryness, dissolve it in 30 ml of methanol, add 20 g of resin, add 210 ml of dichloromethane, and stir for 15 min; after stirring at 40°C for 7 h, cool to room temperature, filter, and wash with methanol. Dry to constant weight. Pour the dried polymer resin into a round bottom flask, add 30ml of methanol, 210ml of dichloromethane, and 2ml of triethylamine, heat to reflux for 7h, filter, wash with methanol, evaporate the filtrate to dryness, and weigh 1.93g. Yield 48.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com