Compositions and methods for combination antiviral therapy
A technology of composition and use, applied in the field of chemically stable combinations, capable of solving problems such as enhanced toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
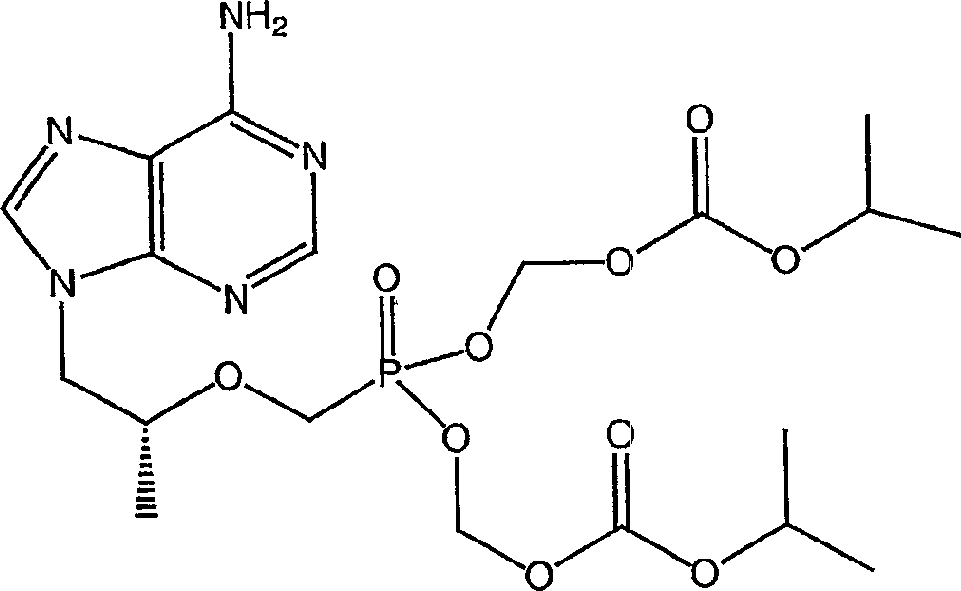
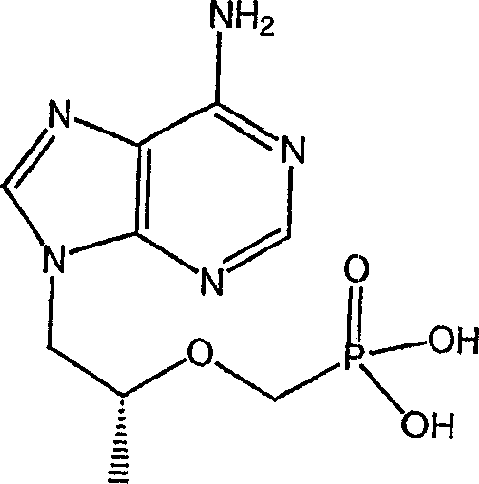
[0209] The following examples further describe and demonstrate specific embodiments falling within the scope of the present invention. Related methods and formulations can generally be found in Remington's Pharmaceutical Sciences (Mack Publishing Co., Easton, PA). The illustrated embodiments are for the purpose of illustration only and therefore should not be construed as limiting since various modifications are possible without departing from the spirit and scope of the invention. The following examples are for illustration only and are not meant to limit the scope of the invention in any way. "Active ingredient" means tenofovir disoproxil fumarate, emtricitabine, or any one of their physiological function derivatives.
[0210] tablet
[0211] Exemplary formulations A, B, C, D, E, and F below were prepared by wet granulating the ingredients with an aqueous solution followed by the addition of the extragranlar component followed by magnesium stearate and compression ....
Embodiment approach
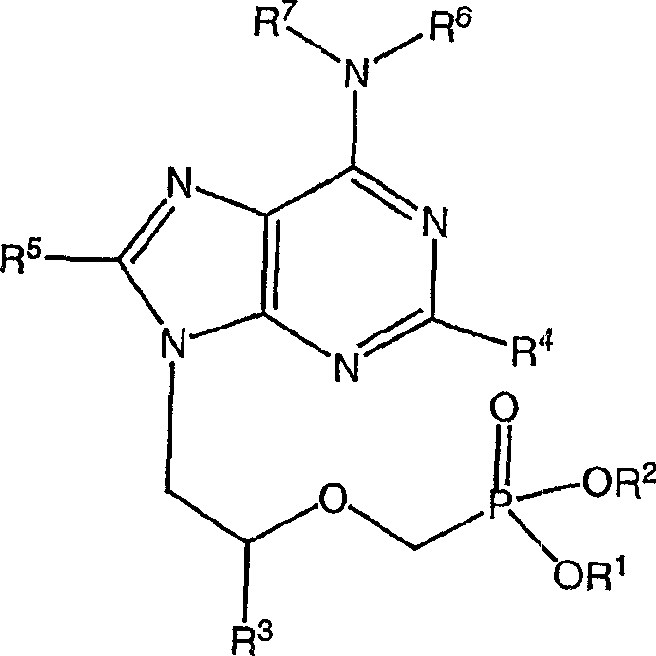
[0358] A1. A pharmaceutical composition, which jointly contains an effective amount of the compound of the following formula or its physiological function derivatives:
[0359]
[0360] where R 1 and R 2 independently selected from H, C 1 -C 6 Alkyl, substituted C 1 -C 6 Alkyl, C 6 -C 20 Aryl, substituted C 6 -C 20 Aryl, C 6 -C 20 Aralkyl, substituted C 6 -C 20 Aralkyl, acyloxymethyl ester -CH 2 OC(=O)R 9 and acyloxymethyl carbonate-CH 2 OC(=O)OR 9 , where R 9 is C 1 -C 6 Alkyl, substituted C 1 -C 6 Alkyl, C 6 -C 20 Aryl and substituted C 6 -C 20 Aryl;
[0361] R 3 Choose from H, C 1 -C 6 Alkyl, substituted C 1 -C 6 Alkyl, or CH 2 OR 8 , where R 8 is C 1 -C 6 Alkyl, C 1 -C 6 Hydroxyalkyl or C 1 -C 6 Haloalkyl;
[0362] R 4 and R 5 independently selected from H, NH 2 , NHR or NR 2 , where R is C 1 -C 6 alkyl; and
[0363] R 6 and R 7 independently selected from H and C 1 -C 6 alkyl;
[0364] and an effective amount of the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com