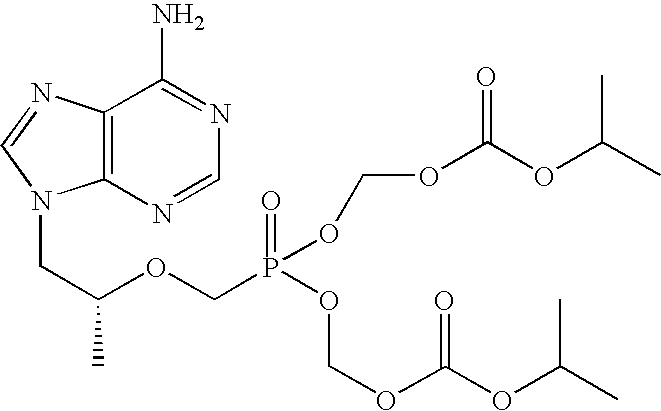
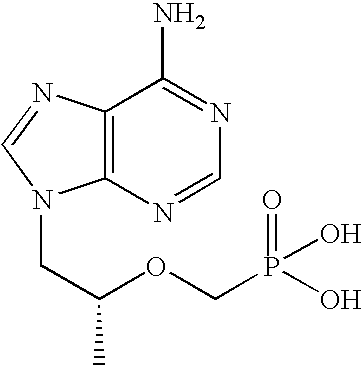
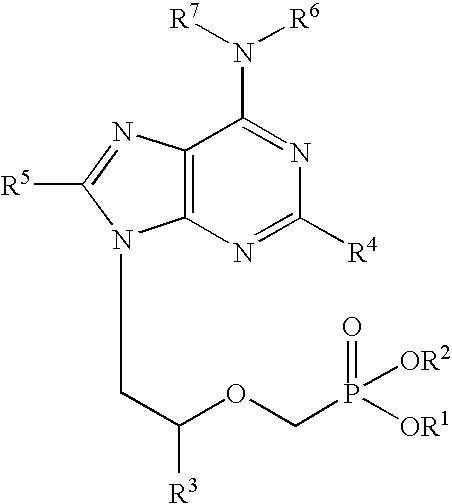
Compositions and methods for combination antiviral therapy
a combination therapy and compound technology, applied in the field of antiviral properties, can solve the problems of affecting the treatment effect of patients, so as to reduce the side effects, improve the effect of patient compliance, and reduce the burden of pills
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0015] While the invention will be described in conjunction with the enumerated claims, it will be understood that they are not intended to limit the invention to those claims. On the contrary, the invention is intended to cover all alternatives, modifications, and equivalents, which may be included within the scope of the present invention as defined by the claims.
Definitions
[0016] Unless stated otherwise, the following terms and phrases as used herein are intended to have the following meanings:
[0017] When tradenames are used herein, applicants intend to independently include the tradename product and the active pharmaceutical ingredient(s) of the tradename product.
[0018] The term “chemical stability” means that the two primary antiviral agents in combination are substantially stable to chemical degradation. Preferably, they are sufficiently stable in physical combination to permit commercially useful shelf life of the combination product. Typically, “chemically stable” means...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight ratio | aaaaa | aaaaa |
| pKa | aaaaa | aaaaa |
| pKa | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com