Method for synthesizing emtricitabine intermediate
A synthesis method and thiolane technology are applied in the synthesis field of emtricitabine intermediates, can solve problems such as affecting the health and safety of operators, high toxicity of raw materials, pollute the environment of the environment, etc., and achieve low production cost, The effect of simplifying the reaction steps and saving production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
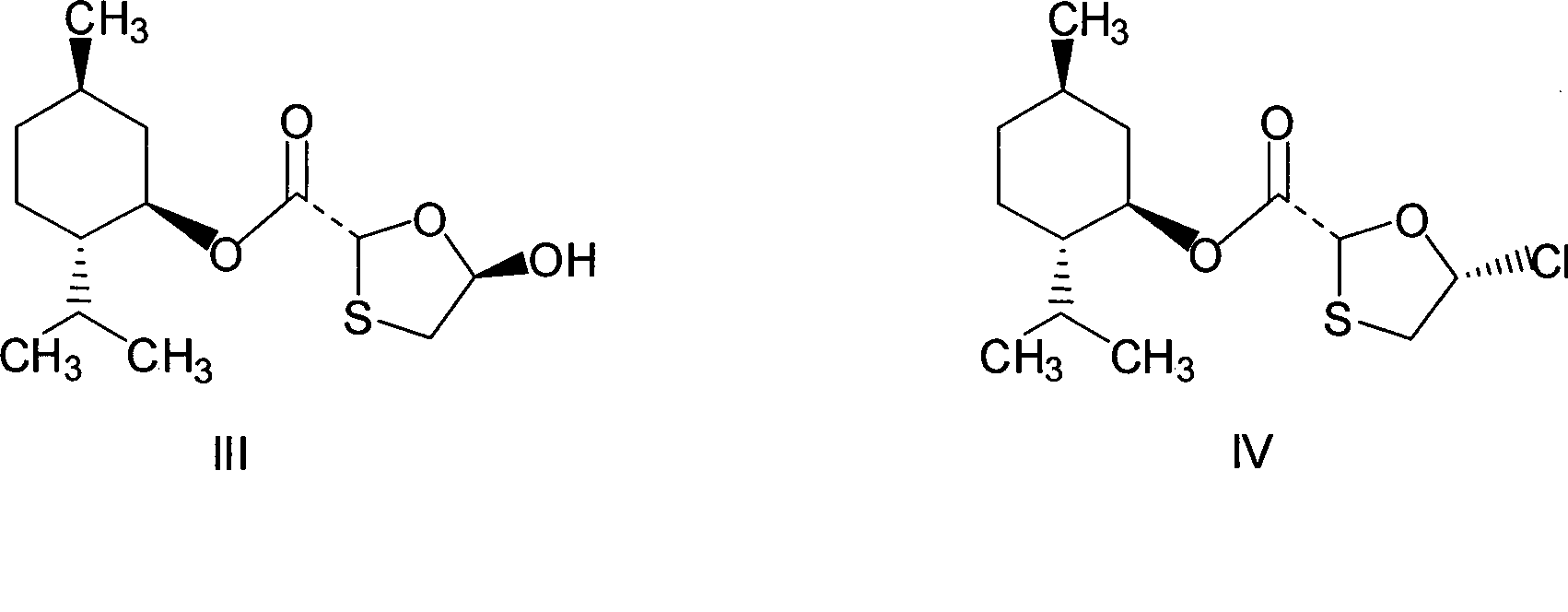
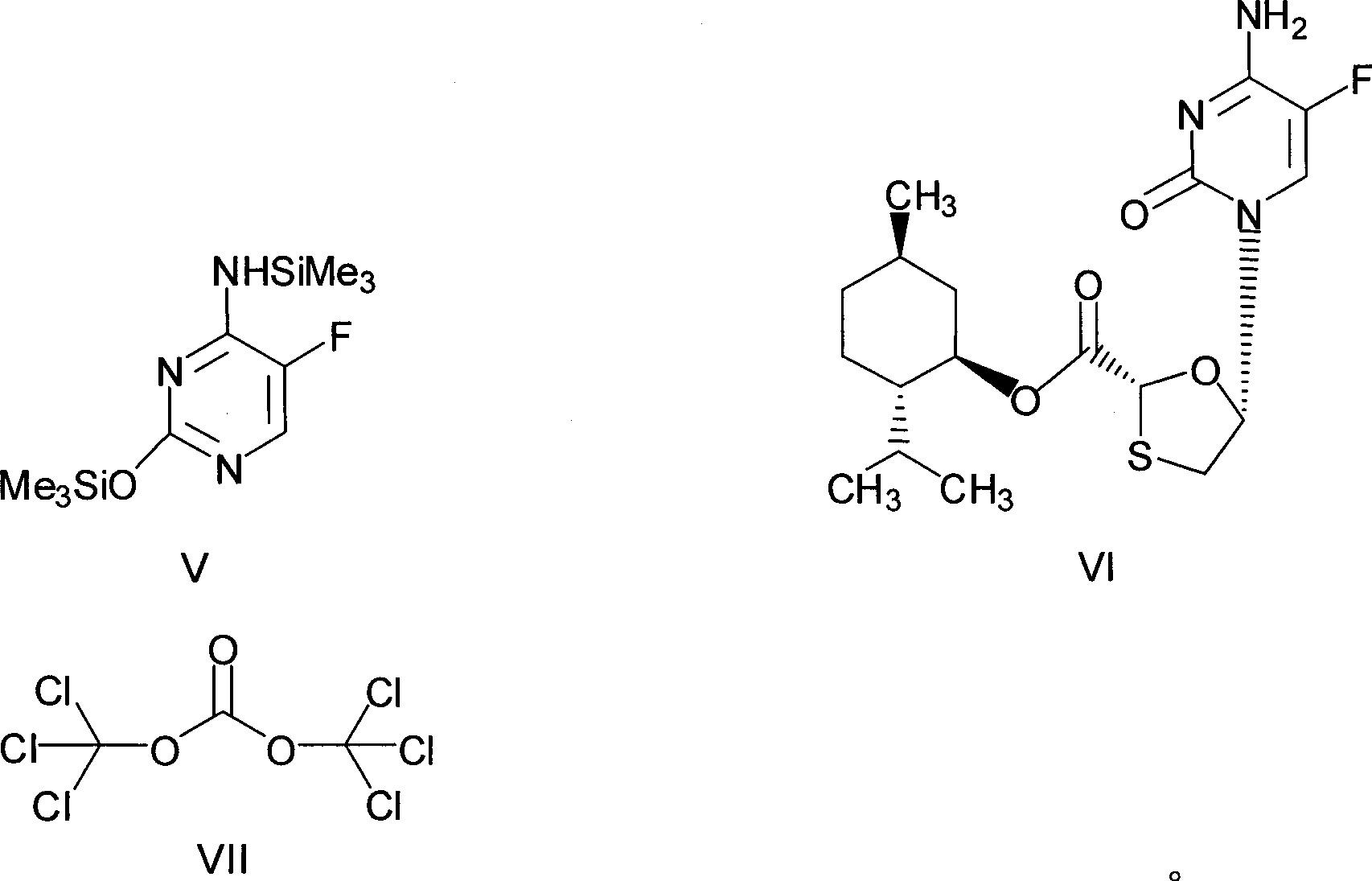
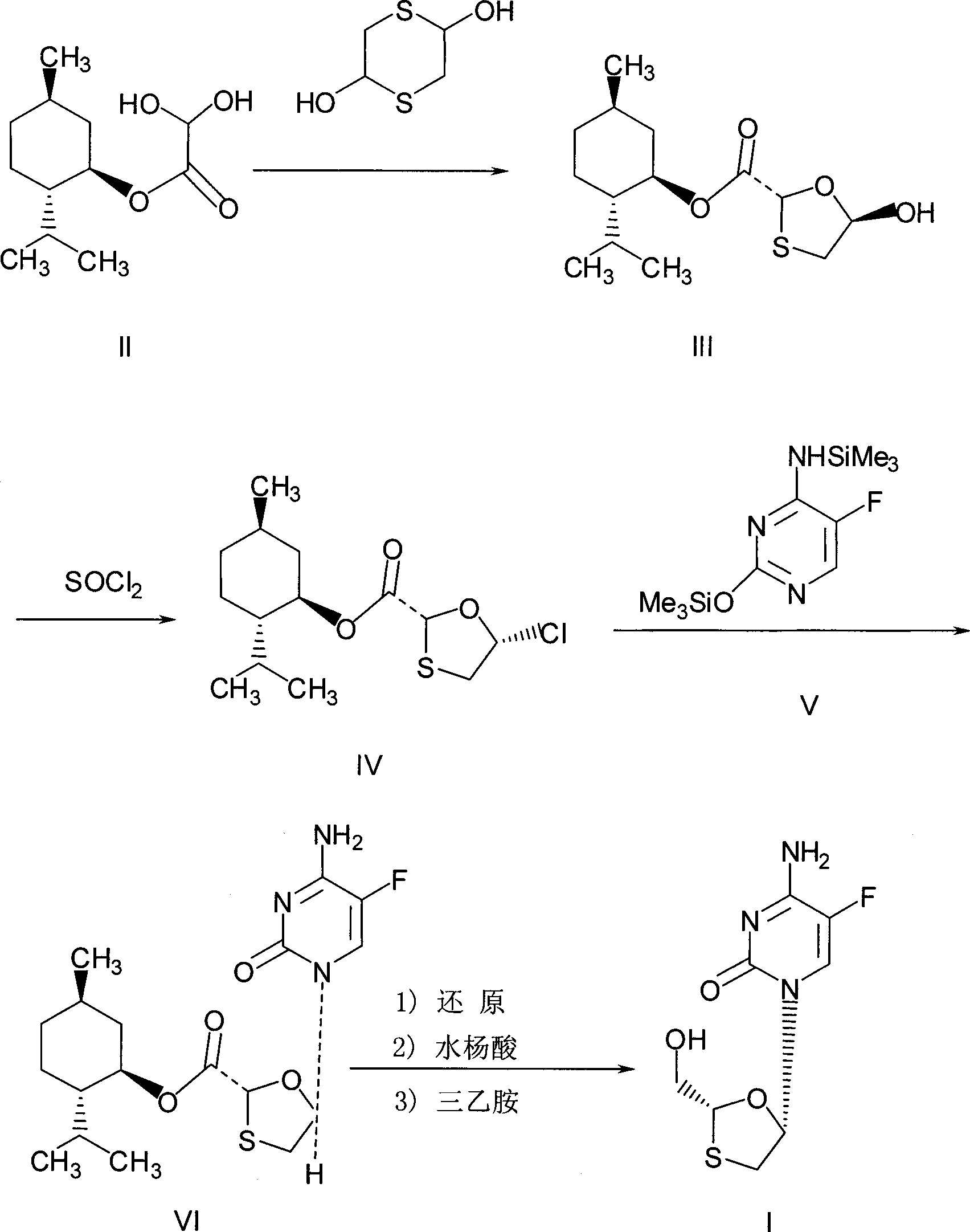
[0027] Preparation of chlorinated compounds: in a 250mL three-necked flask, add 28.8 grams of (2R, 5R)-5-hydroxyl-1,3-oxathiolane-2-carboxylic acid-L-menthyl ester (compound III) ( 0.1mol), N, N-dimethylformamide 7.3g, 100mL dichloromethane, and keep stirring until compound III dissolves. Cool to about 0°C, control the temperature at 5-10°C, add dropwise a mixed solution of 11.9 grams (0.04mol) of bis(trichloromethyl)carbonate in 50mL of dichloromethane, and slowly heat up to 30 -35°C, keep warm for 2 hours. The resulting reaction solution is a chloride (compound IV) solution, ready for use.
[0028] Preparation of N,O-bis(trimethylsilyl)5-fluorocytosine: In a 500mL three-neck flask, add 12.9 grams (0.1mol) of 5-fluorocytosine, 24.5 grams of hexamethyldisilazane, 100mL toluene, 3 Drop methanesulfonic acid, heat to reflux for 2 hours, until the solution is clear, then cool slightly, this is the toluene solution of N,O-bis(trimethylsilyl)5-fluorocytosine.
[0029] Preparation...
Embodiment 2
[0032] Preparation of chlorinated compounds: in a 250mL three-necked flask, add 28.8 grams of (2R, 5R)-5-hydroxyl-1,3-oxathiolane-2-carboxylic acid-L-menthyl ester (compound III) ( 0.1mol), N, N-dimethylformamide 7.3g, 100mL dichloromethane, and keep stirring until compound III dissolves. Cool to about 0°C, control the temperature at 5-10°C, add dropwise a mixed solution of 14.9 grams (0.05mol) of bis(trichloromethyl)carbonate in 50mL of dichloromethane, and slowly heat up to 30 -35°C, keep warm for 2 hours. The resulting reaction solution is a chloride (compound IV) solution, ready for use.
[0033] Preparation of N, O-bis(trimethylsilyl) 5-fluorocytosine: same as Example 1.
[0034] Preparation of (2R, 5S)-5-(5'-fluoro-cytosine-1-yl)-1,3-oxathiolane-2-carboxylic acid-L-menthyl ester: same as Example 1
[0035] Post-treatment: same as Example 1, to obtain 27.1 g of white solid product, HPLC purity: 99.50%, chiral purity: 99.35%, melting point: 220.1-220.9°C, yield: 67.9%. ...
Embodiment 3
[0036] Example 3 One Pot Method
[0037] In a 500mL three-necked flask, add 12.9g (0.1mol) of 5-fluorocytosine, 24.5g of hexamethyldisilazane, 100mL of toluene, and 3 drops of methanesulfonic acid, heat and reflux for 2 hours until the solution is clear, this is N, O-bis(trimethylsilyl)5-fluorocytosine in toluene. Slightly cold, add 7.3g (0.1mol) of N,N-dimethylformamide, 12g of triethylamine, at a temperature of about 20-25°C, slowly add the (2R,5R)-5-hydroxy -1,3-oxathiolane-2-carboxylic acid-L-menthyl ester (compound III) 28.8 grams (0.1mol) and bis(trichloromethyl)carbonate 11.9 grams (0.04mol) were dissolved in 150mL of the mixed solution of dichloromethane, after dripping, keep warm for 2 hours, then slowly raise the temperature to 40-45°C, and react for 8 hours. Stop responding.
[0038] Post-treatment: Pour the reaction solution into a mixed solution containing 100g of water, 12g of triethylamine, and 50g of n-hexane, stir for 8 hours, filter, and rinse the resultin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com