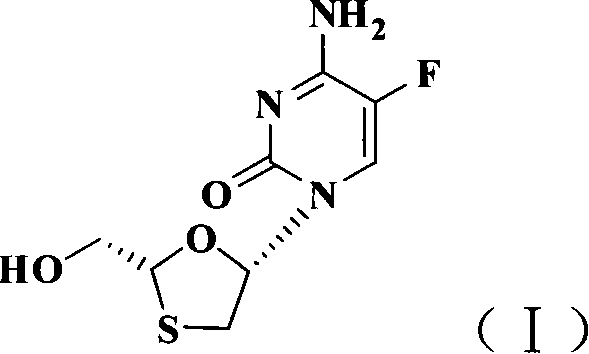
Non-enantioselective prepn process of emtricitabine
A technology of emtricitabine and diastereomer, applied in the field of drug preparation, can solve the problems that the optical purity of the product does not reach the medicinal standard, is not suitable for industrial production, and the yield is not high, and achieves huge social and economic benefits, The effect of low cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] The invention will be further described below through specific examples.
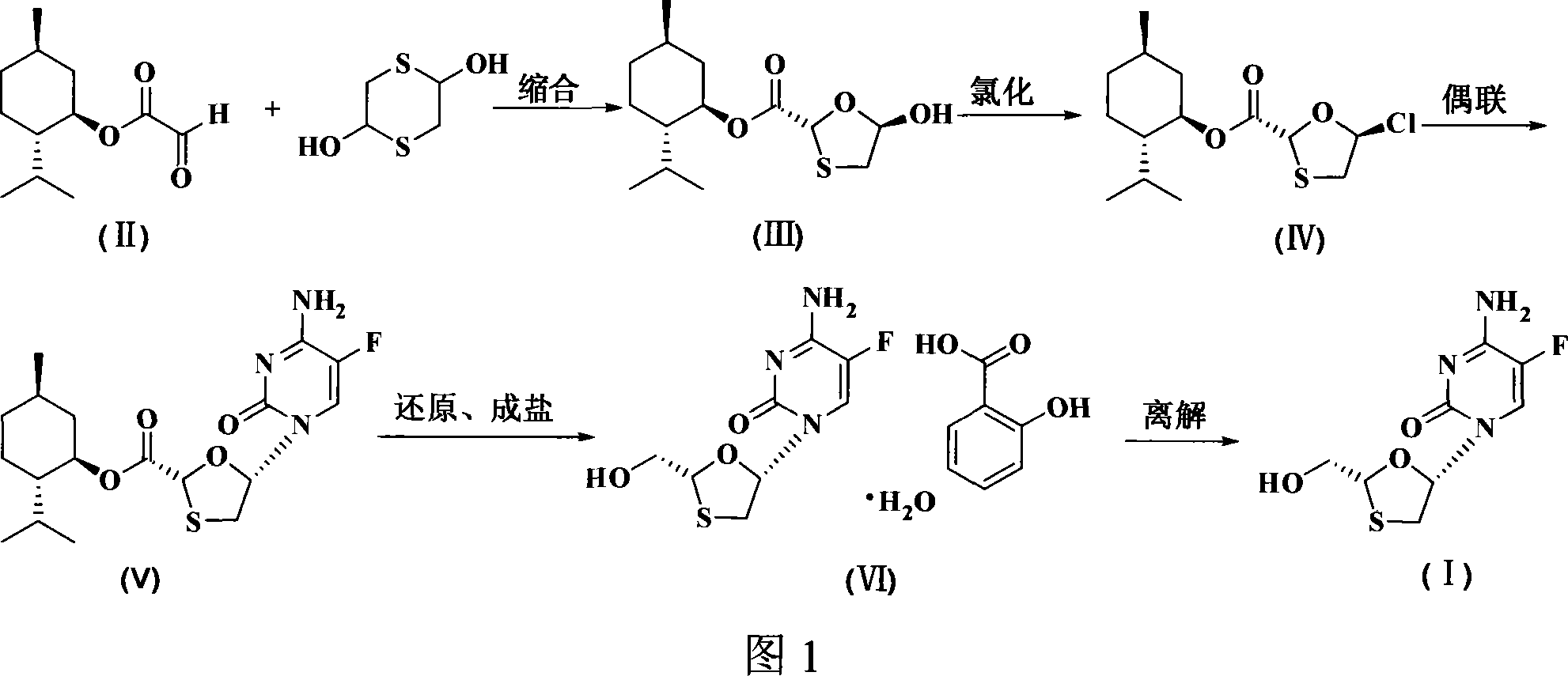
[0028] As shown in Figure 1, the diastereoselective preparation method of emtricitabine is synthesized through the following steps:
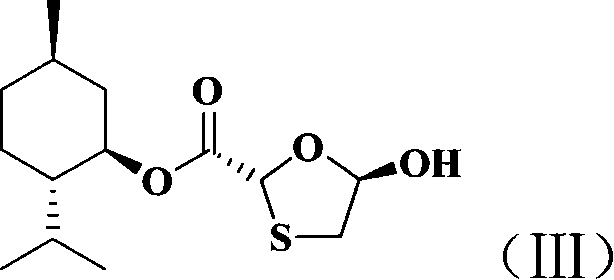
[0029] 1. Preparation of trans-5-hydroxy-1,3-oxathiolane-2-carboxylic acid-L-menthol ester (III).
[0030]
[0031] Under nitrogen protection, mix glyoxylic acid-L-menthyl ester (100g, 0.43mol), dithiothiane (33g, 0.215mol) and 400ml toluene, heat to dissolve, add p-toluenesulfonic acid (2.22g, 0.0129mol), the temperature was raised to react, and the water in the reaction system was separated with a water trap until no more water came out, about 5 hours. Cool, add triethylamine to adjust the pH to 7-8, wash the organic layer with deionized water, remove the solvent from the obtained organic layer, add petroleum ether, let stand at room temperature, filter, and dry to obtain 110 g of white powdery solid, yield 88.7% . 1 H-NMR (CDCl 3 )δ: 0.71~2.09(m, 18H, 2’~...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com