Patents
Literature
65 results about "Amoebocyte lysate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
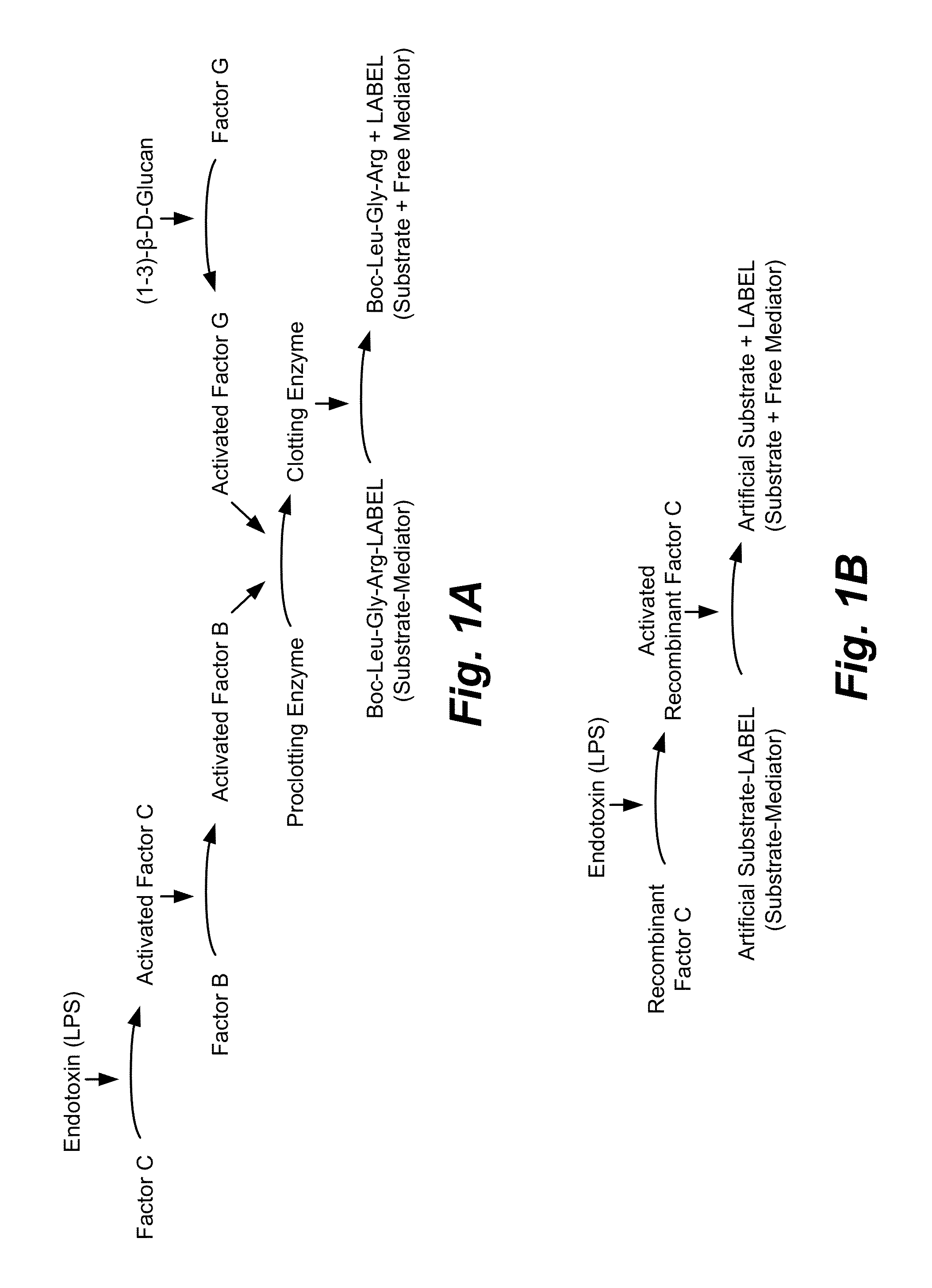
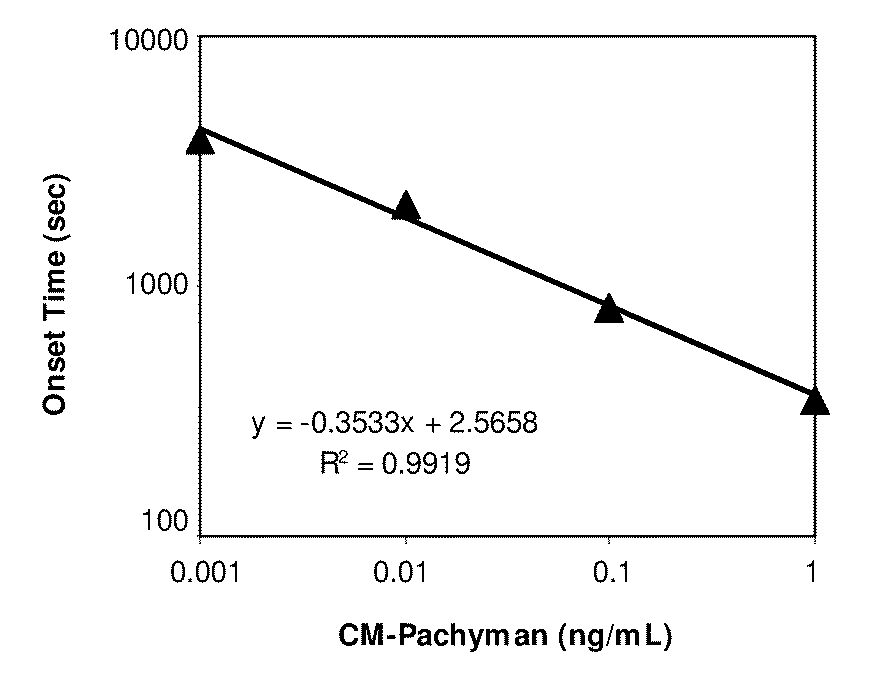
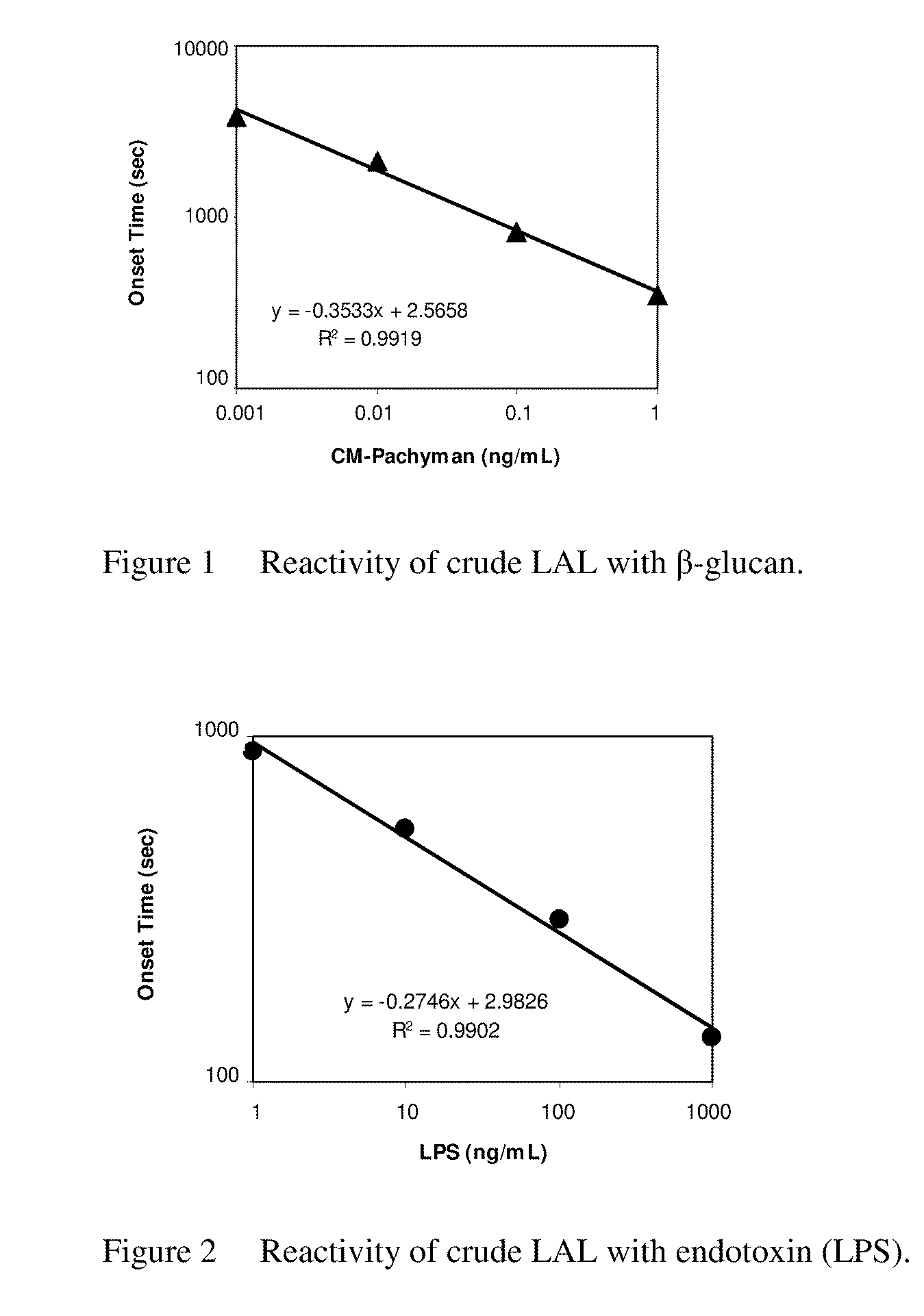
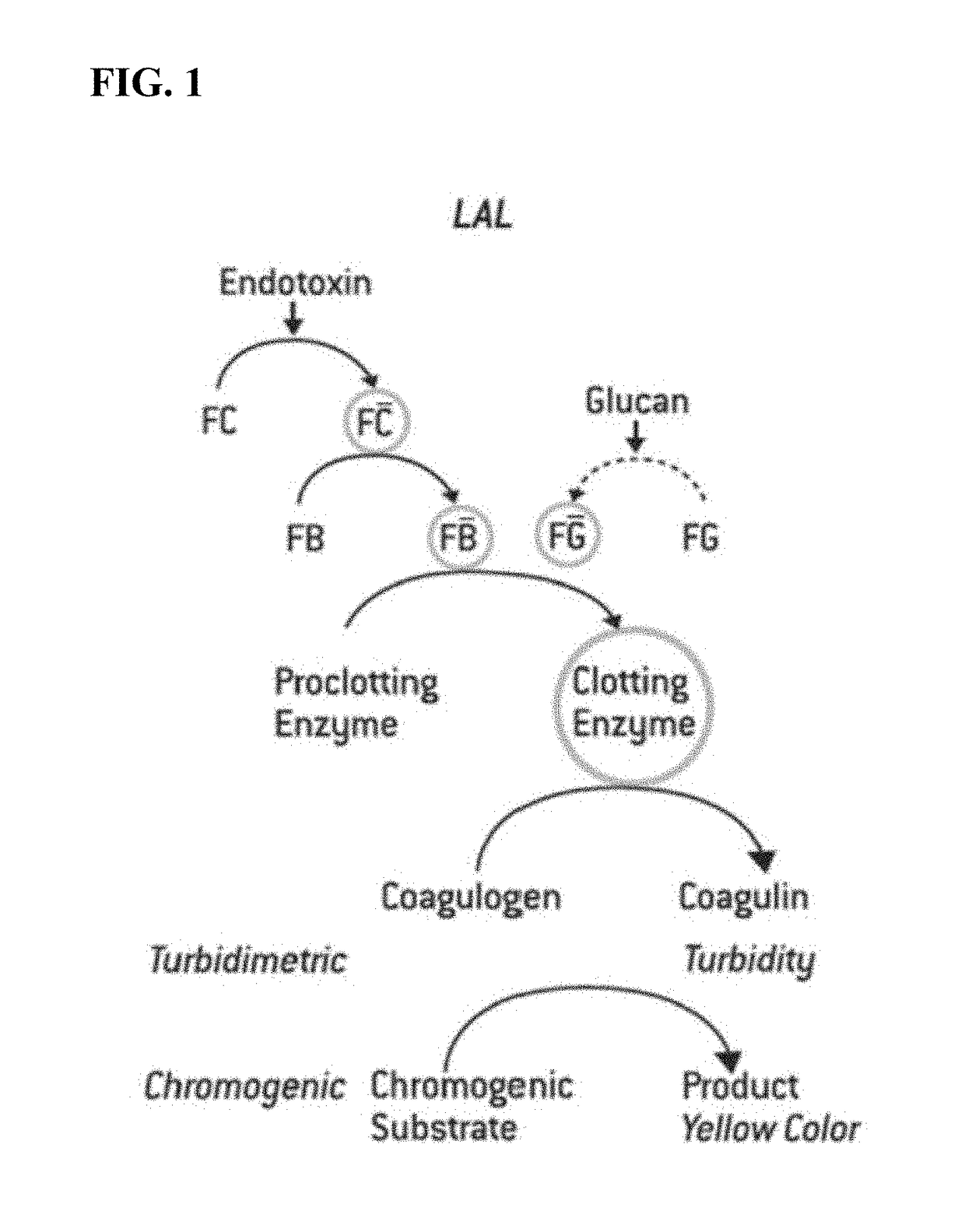
Limulus amebocyte lysate (LAL) is an aqueous extract of blood cells (amoebocytes) from the Atlantic horseshoe crab, Limulus polyphemus. LAL reacts with bacterial endotoxin lipopolysaccharide (LPS), which is a membrane component of gram-negative bacteria.
Detection of pyrogen and other impurities in water
InactiveUS20010040130A1Improve stabilityHigh sensitivityIon-exchanger regenerationScattering properties measurementsRefractive indexAmoebocyte lysate
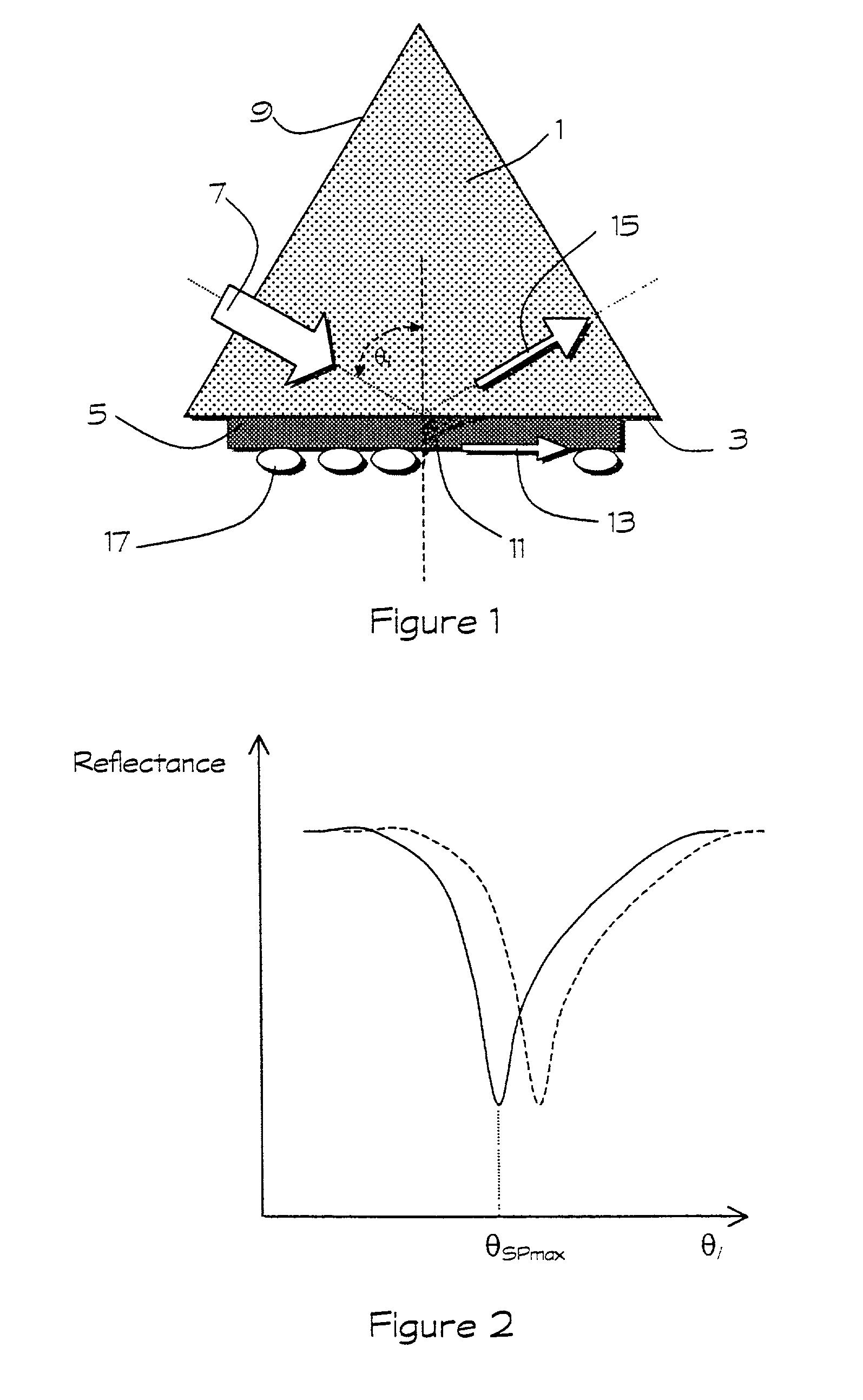
High purity water, particularly that intended for the pharmaceutical or electronics industry, is analyzed for the presence of pyrogen or other impurities by causing the water to come into contact with a direct affinity sensor, which may be a surface plasmon resonance (SPR) device or other sensor relying on an evanescent wave phenomenon. A property of the surface-refractive index in the case of SPR-changes on the binding of impurity, thereby enabling impurity to be detected. The invention overcomes the cumbersome nature and batch-to-batch variability of the conventional in vivo tests as well as the in vitro Limulus Amoebocyte Lysate (LAL) assay and for the first time allows the continuous or real time monitoring of high purity water for pyrogen.
Owner:THE LORCH FOUND +1
Construction method of cell model for detecting pyrogens, cell model and pyrogen detection kit
ActiveCN106148286AImprove stabilityIncreased sensitivityCell receptors/surface-antigens/surface-determinantsCulture processWestern blotCytokine
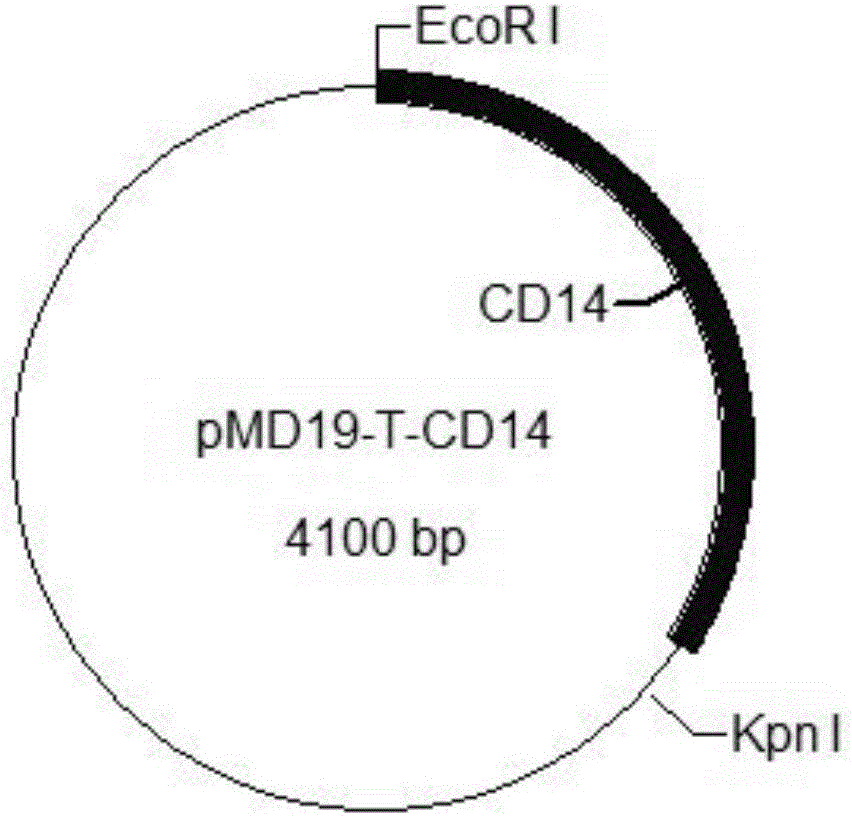
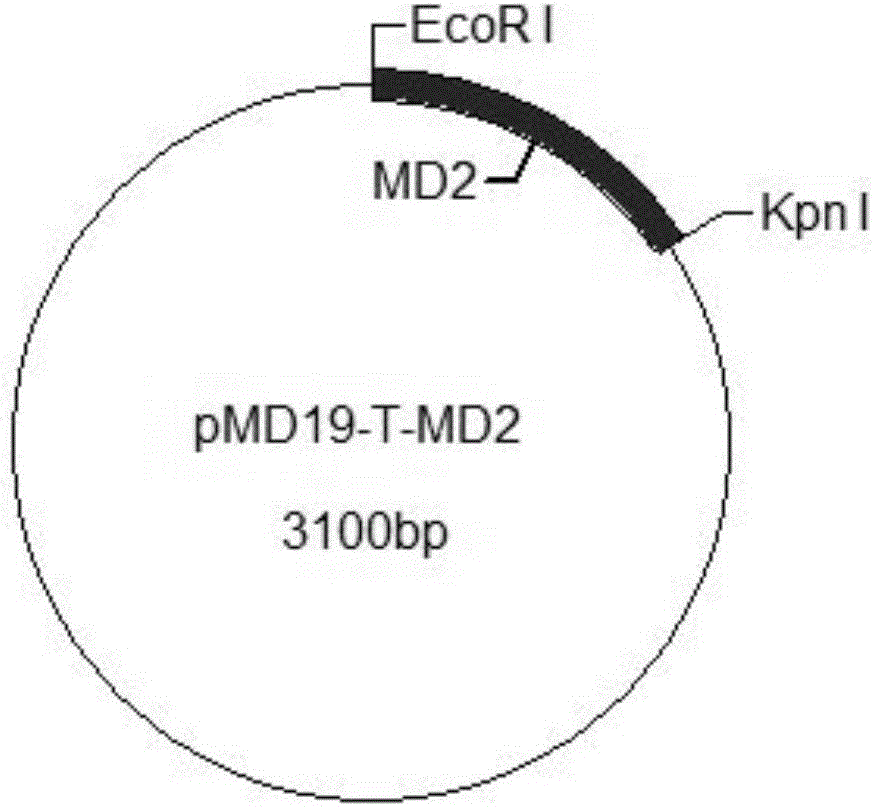
The invention provides a construction method of a cell model for detecting pyrogens, the cell model and a pyrogen detection kit. The cell model utilizes specific locations of CRISPR / CAS9 induced genomes to form double-bond fission, TLR4 and CD14-MD2 are knocked into two chromosomes of a cell line respectively by the aid of the homologous recombination repair principle, green fluorescence GFP and red fluorescence RFP are respectively used for tracing finally successfully constructed TLR4 / CD14 / MD2 fixed-point knocked-in fluorescent tracer cell models, and the LPS stimulating cell model can detect release of IL-6 and TNF-a cytokines by means of ELISA, Western Blot, mass spectrum and immunomagnetic beads. The cell model is good in stability and high in sensitivity, and the lowest detectable limit can reach 0.005EU / mL and is far lower than 0.025EU / mL of the Tachypleus Amebocyte Lysate method.
Owner:牛刚
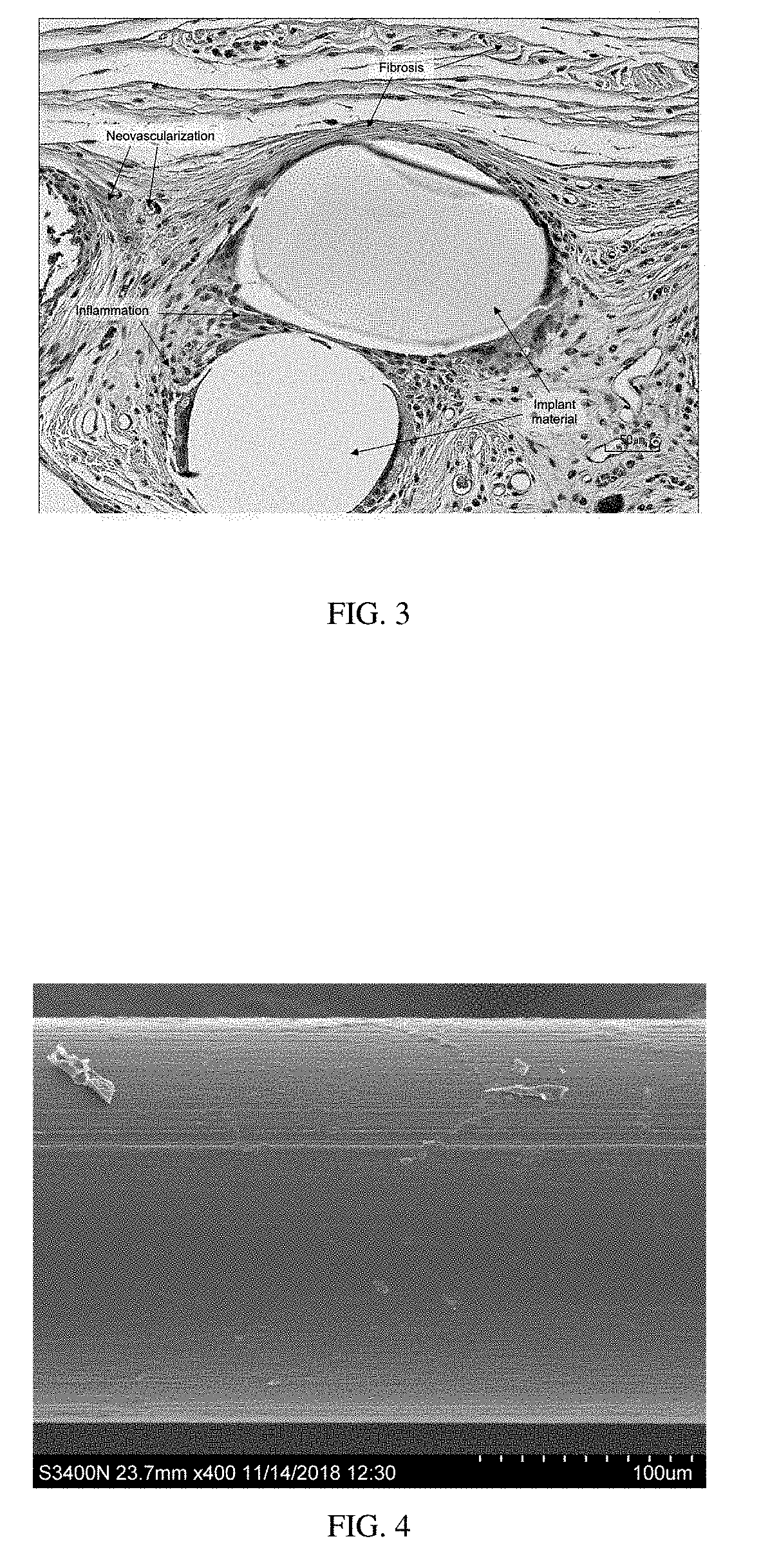
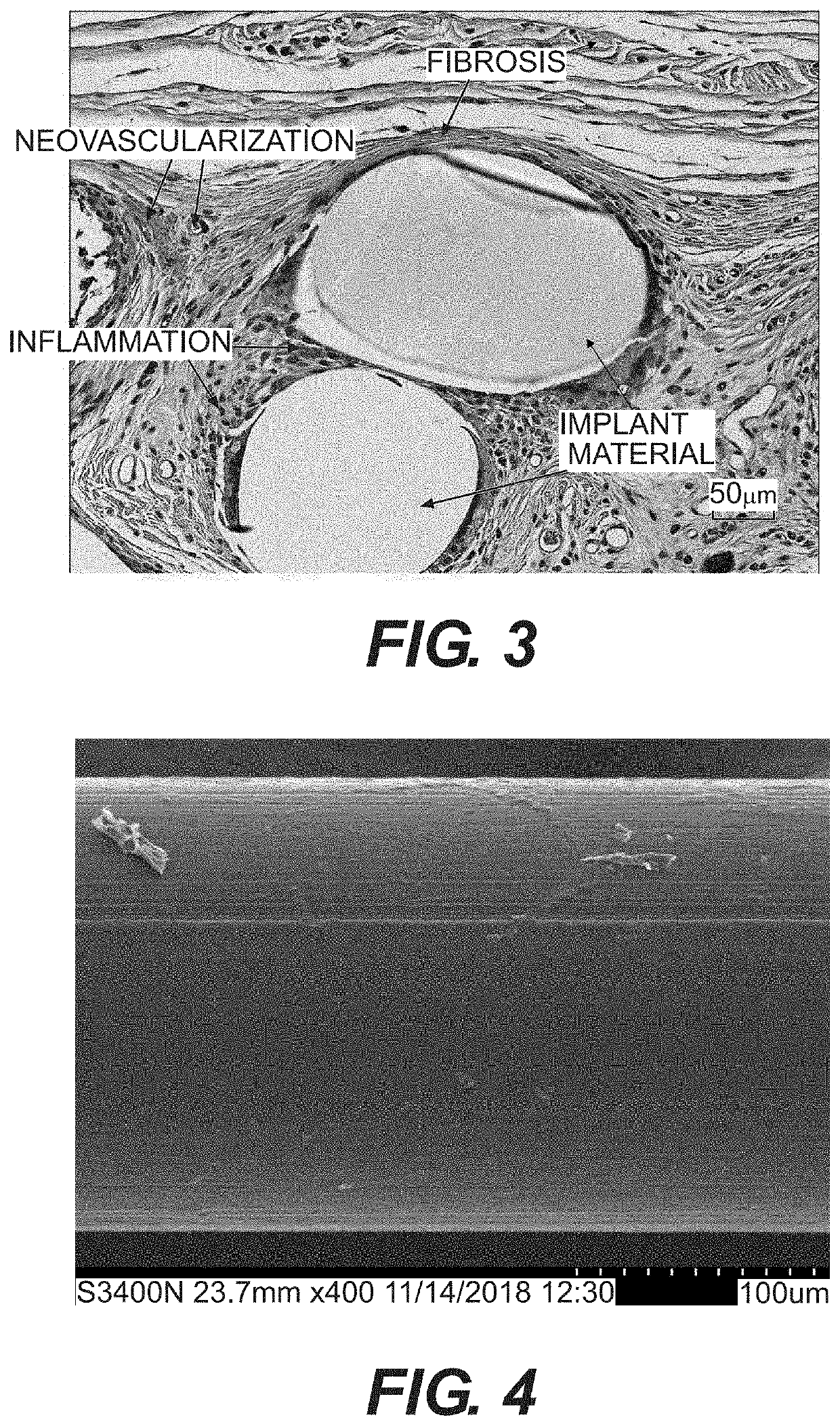
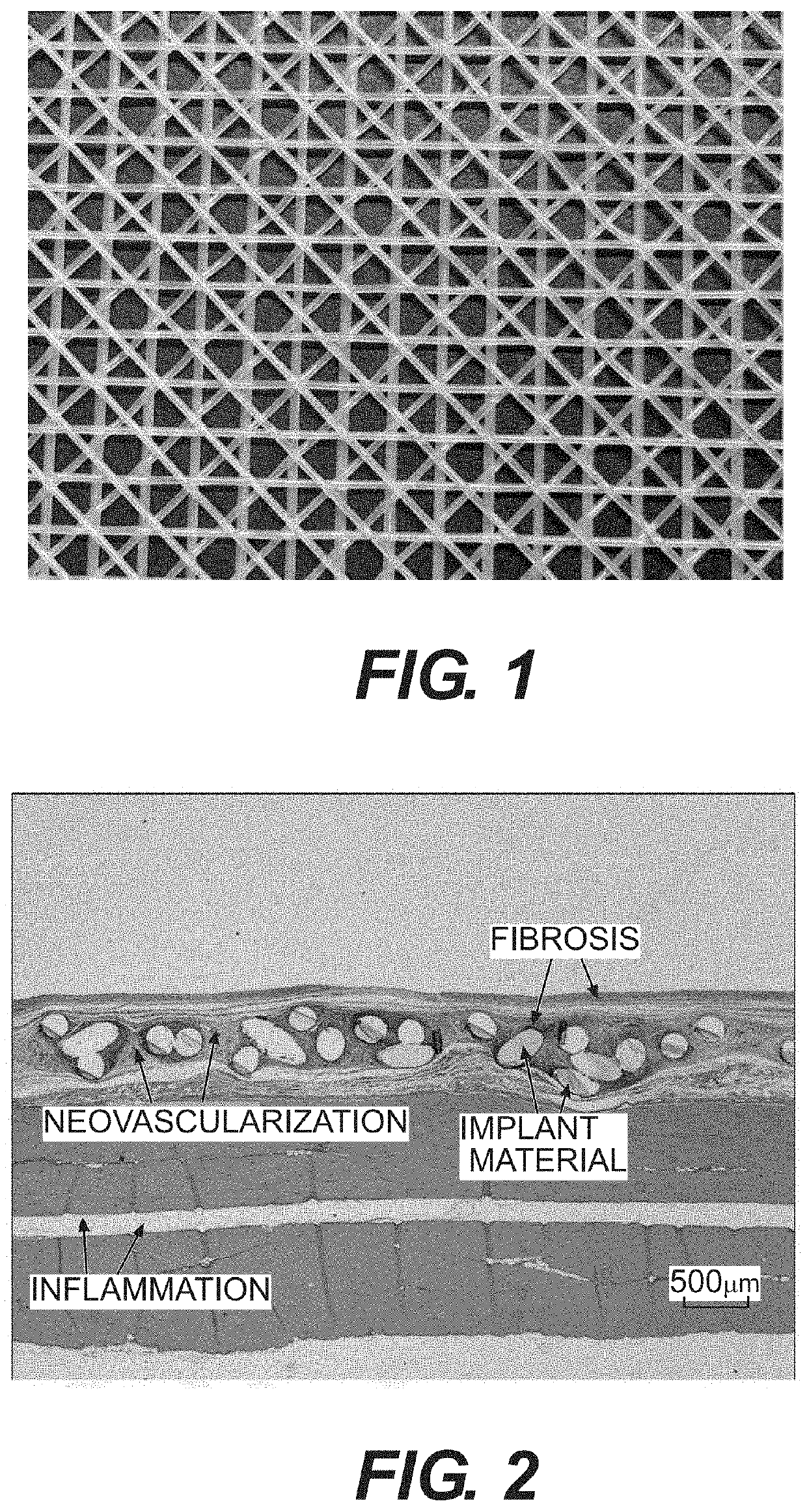
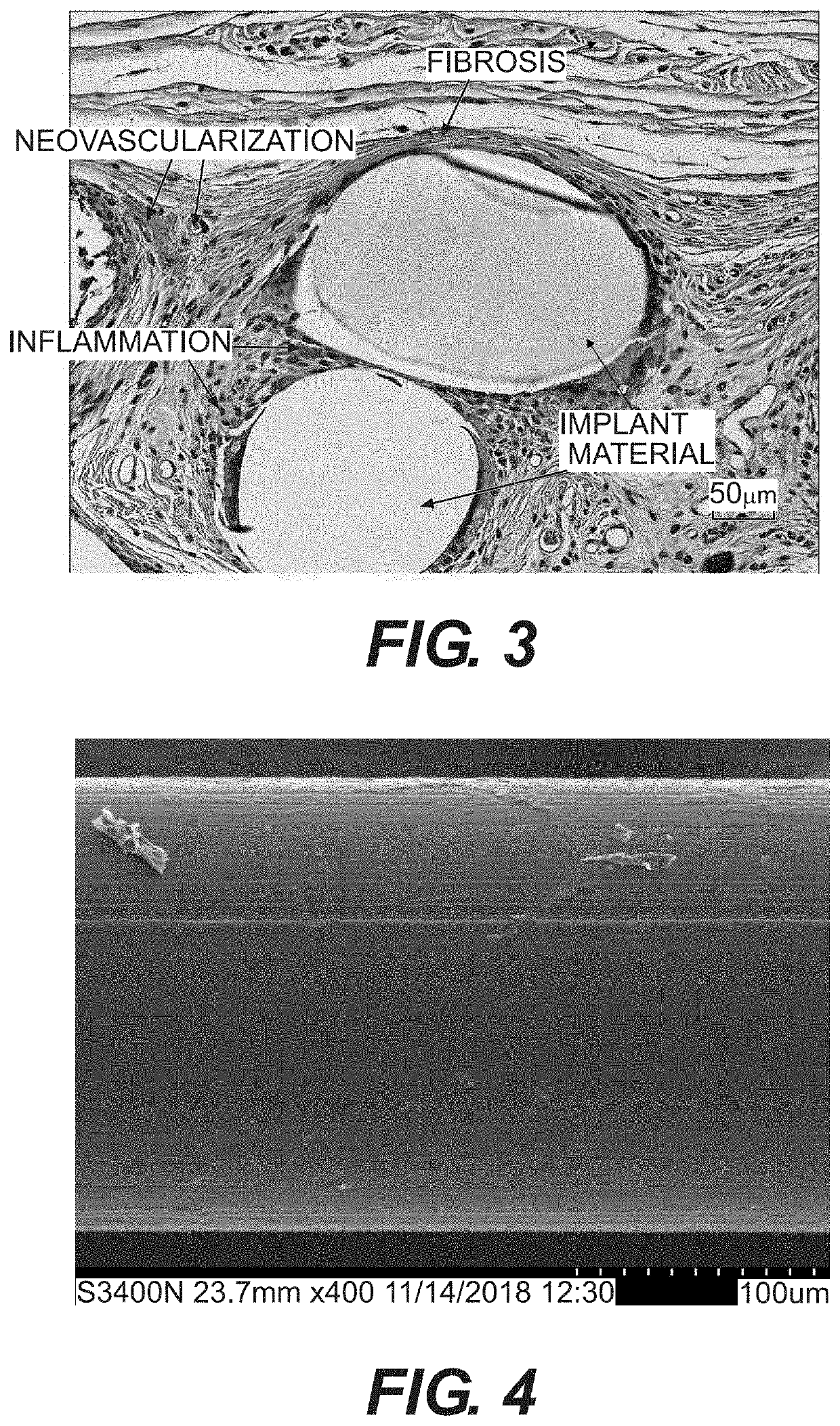
Surgial mesh implants containing poly(butylene succinate) and copolymers thereof
PendingUS20190269817A1Inhibition of colonizationReduce and prevent occurrenceSuture equipmentsMammary implantsFiberLimulus amebocyte lysate
Resorbable implants comprising poly(butylene succinate) and copolymers thereof have been developed. The implants implants are preferably sterilized, and contain less than 20 endotoxin units per device as determined by the limulus amebocyte lysate (LAL) assay, and are particularly suitable for use in procedures where prolonged strength retention is necessary, and can include one or more bioactive agents. The implants may be made from fibers and meshes of poly(butylene succinate) and copolymers thereof, or by 3d printing, and the fibers may be oriented. Coverings and receptacles made from forms of poly(butylene succinate) and copolymers thereof have also been developed for use with cardiac rhythm management devices and other implantable devices. These coverings and receptacles may be used to hold, or partially / fully cover, devices such as pacemakers and neurostimulators. The coverings and receptacles are made from meshes, webs, lattices, non-wovens, films, fibers, and foams, and contain antibiotics such as rifampin and minocycline.
Owner:TEPHA INC
Amperometric detection of limulus amebocyte lysate activation by endotoxin and/or 1-3-beta-d-glucan
InactiveUS20150060272A1Immobilised enzymesBioreactor/fermenter combinationsBeta d glucanBiological activation
Devices to detect detecting endotoxin (lipopolysaccharide or LPS) and / or 1-3-β-D-glucan (beta glucan or BG) using substrates including electrogenic mediators for amperometric detection are provided herein. Substrates include an amine group as well as one or more substituted organic rings, such as a phenol. Devices include cartridges for detecting LPS or BG simultaneously using dual detection techniques.
Owner:NANOMIX
Portable device for nmr based analysis of rheological changes in liquid samples
InactiveUS20150130463A1Laboratory glasswaresAnalysis using nuclear magnetic resonanceEngineeringActuator
The invention features a portable device for monitoring changes in the rheological state of multiple samples by NMR based measurement. The rheological changes can be, for example, gelation occurring during limulus amoebocyte lysate (LAL) testing for endotoxins or blood coagulation. The inventive method entails organization of individual NMR tubes in a disposable cartridge where the tubes are connected through a top cover, provided with movable actuators to push the reagent in the reagent compartments of the individual chambers to the reaction chambers in a predetermined sequence.
Owner:T2 BIOSYST
Oriented implants containing poly(butylene succinate) and copolymer, and methods of use thereof
PendingUS20190269816A1Inhibition of colonizationReduce and prevent occurrenceSuture equipmentsMammary implantsFiberLimulus amebocyte lysate
Resorbable implants comprising poly(butylene succinate) and copolymers thereof have been developed. The implants implants are preferably sterilized, and contain less than 20 endotoxin units per device as determined by the limulus amebocyte lysate (LAL) assay, and are particularly suitable for use in procedures where prolonged strength retention is necessary, and can include one or more bioactive agents. The implants may be made from fibers and meshes of poly(butylene succinate) and copolymers thereof, or by 3d printing, and the fibers may be oriented. Coverings and receptacles made from forms of poly(butylene succinate) and copolymers thereof have also been developed for use with cardiac rhythm management devices and other implantable devices. These coverings and receptacles may be used to hold, or partially / fully cover, devices such as pacemakers and neurostimulators. The coverings and receptacles are made from meshes, webs, lattices, non-wovens, films, fibers, and foams, and contain antibiotics such as rifampin and minocycline.
Owner:TEPHA INC
Articles of poly(butylene succinate) and copolymers thereof
ActiveUS20200390933A1Reduce usageInhibition of colonizationSuture equipmentsInternal osteosythesisPolymer scienceActive agent
Resorbable implants, coverings and receptacles comprising poly(butylene succinate) and copolymers thereof have been developed. The implants are preferably sterilized, and contain less than 20 endotoxin units per device as determined by the limulus amebocyte lysate (LAL) assay, and are particularly suitable for use in procedures where prolonged strength retention is necessary, and can include one or more bioactive agents. The implants may be made from fibers and meshes of poly(butylene succinate) and copolymers thereof, or by 3d printing molding, pultrusion or other melt or solvent processing method. The implants, or the fibers preset therein, may be oriented. These coverings and receptacles may be used to hold, or partially / fully cover, devices such as pacemakers and neurostimulators. The coverings, receptacles and implants described herein, may be made from meshes, webs, lattices, non-wovens, films, fibers, foams, molded, pultruded, machined and 3D printed forms.
Owner:TEPHA INC
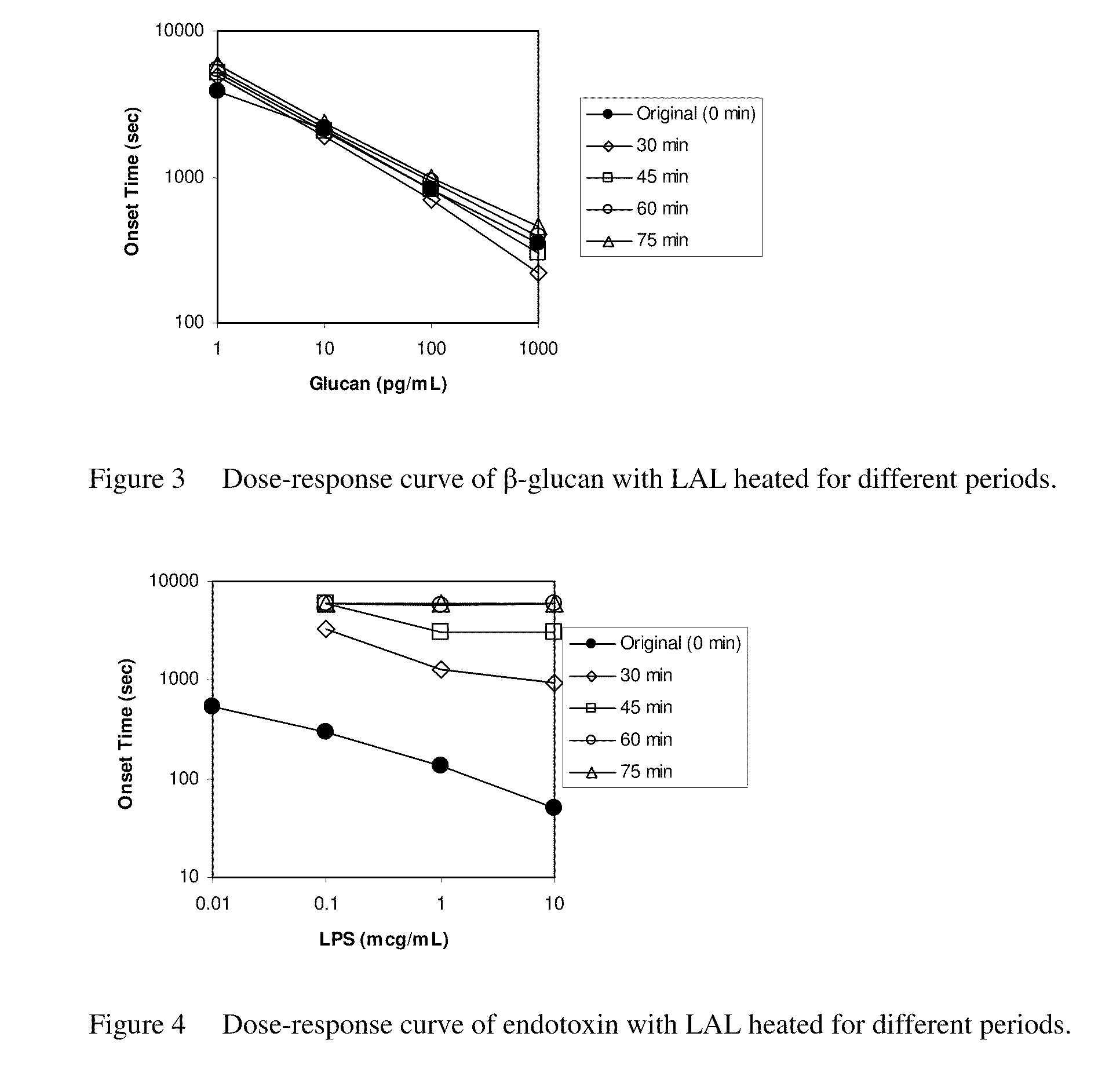
Heat-Treated Limulus Amebocyte Lysates
ActiveUS20100330597A1Reduced responseLose sensitivityMicrobiological testing/measurementBiological material analysisLimulus amebocyte lysateCell lysates
Owner:CHARLES RIVER LAB INC
Droplet microfluidic device and methods of sensing the result of assay therein
A method of determining the result of an assay in a microfluidic device includes the steps of: dispensing a sample droplet onto a first portion of an electrode array of the microfluidic device; dispensing a reagent droplet onto a second portion of the electrode array of the microfluidic device; controlling actuation voltages applied to the electrode array to mix the sample droplet and the reagentdroplet into a product droplet; sensing a dynamic property of the product droplet; and determining an assay of the sample droplet based on the sensed dynamic property. The dynamic property is a physical property of the product droplet that influences a transport property of the product droplet on the electrode array. Example dynamic properties of the product droplet include the moveable state, split-able state, and viscosity based on droplet properties. The method may be used to perform an amoebocyte lysate (LAL) assay.
Owner:SHARP LIFE SCI EU LTD
Droplet microfluidic device and methods of sensing the results of an assay therein
ActiveCN107923914AEasy to useLow costLaboratory glasswaresMaterial analysisSeparable stateEngineering
A method of determining the result of an assay in a microfluidic device includes the steps of: dispensing a sample droplet onto a first portion of an electrode array of the microfluidic device; dispensing a reagent droplet onto a second portion of the electrode array of the microfluidic device; controlling actuation voltages applied to the electrode array to mix the sample droplet and the reagentdroplet into a product droplet; sensing a dynamic property of the product droplet; and determining an assay of the sample droplet based on the sensed dynamic property. The dynamic property is a physical property of the product droplet that influences a transport property of the product droplet on the electrode array. Example dynamic properties of the product droplet include the moveable state, split-able state, and viscosity based on droplet properties. The method may be used to perform an amoebocyte lysate (LAL) assay.
Owner:SHARP LIFE SCI EU LTD +1
Tissue restoration structure derived from fish scales
InactiveCN102327646AHigh mechanical strengthSimplified processing stepsProsthesisBiological bodyTissue repair
The invention relates to a tissue restoration structure derived from fish scales. The tissue restoration structure comprises a base material which has the characteristics of good biological compatibility and biological decomposability and is formed by the fish scales processed by proper steps, and the special microchannel structure is in favor of cell attachment and proliferation. Before using the tissue restoration structure in an organism (such as a human body), the LAL (limulus amebocyte lysate) test value in the tissue restoration structure must be less than 1000Eu / ml, and preferably less than 200Eu / ml; and if the tissue restoration structure is used as special medicinal equipment, the LAL test value must be less than 50Eu / ml.
Owner:BODY ORGAN BIOMEDICAL CORP
Method for amplifying antibody marking signal or nucleic acid probe marking signal
InactiveCN103235116AImprove stabilityNo radioactive contaminationBiological testingNucleic Acid ProbesMicrobiology
The invention discloses a method for amplifying antibody marking signal or nucleic acid probe marking signal, and the main scheme is that: lipopolysaccharide of gram negative bacteria and an antibody are crosslinked, or fungi (1,3)-beta-D-glucan of fungi cell wall and the antibody or a nucleic acid probe are crosslinked, and then tachypleus amebocyte lysate and the gram negative bacteria lipopolysaccharide or fungi cell wall (1,3)-beta-D-glucan which is crosslinked with the antibody are specifically reacted, thereby improving the sensitivity of the antibody or nucleic acid probe detection. The system for amplifying antibody marking signal or nucleic acid probe marking signal can detect object substrates lower than pg level content.
Owner:FUZHOU UNIV
Medical devices containing compositions of poly(butylene succinate) and copolymers thereof
PendingUS20200390944A1Inhibition of colonizationReduce and prevent occurrenceSuture equipmentsInternal osteosythesisActive agentNonwoven fabric
Resorbable implants, coverings and receptacles comprising poly(butylene succinate) and copolymers thereof have been developed. The implants are preferably sterilized, and contain less than 20 endotoxin units per device as determined by the limulus amebocyte lysate (LAL) assay, and are particularly suitable for use in procedures where prolonged strength retention is necessary, and can include one or more bioactive agents. The implants may be made from fibers and meshes of poly(butylene succinate) and copolymers thereof, or by 3d printing molding, pultrusion or other melt or solvent processing method. The implants, or the fibers preset therein, may be oriented. These coverings and receptacles may be used to hold, or partially / fully cover, devices such as pacemakers and neurostimulators. The coverings, receptacles and implants described herein, may be made from meshes, webs, lattices, non-wovens, films, fibers, foams, molded, pultruded, machined and 3D printed forms.
Owner:TEPHA INC
Medical devices containing poly(butylene succinate) and copolymers thereof
PendingUS20210047484A1Reduce usageInhibition of colonizationSuture equipmentsInternal osteosythesisActive agentNonwoven fabric
Resorbable implants, coverings and receptacles comprising poly(butylene succinate) and copolymers thereof have been developed. The implants are preferably sterilized, and contain less than 20 endotoxin units per device as determined by the limulus amebocyte lysate (LAL) assay, and are particularly suitable for use in procedures where prolonged strength retention is necessary, and can include one or more bioactive agents. The implants may be made from fibers and meshes of poly(butylene succinate) and copolymers thereof, or by 3d printing molding, pultrusion or other melt or solvent processing method. The implants, or the fibers preset therein, may be oriented. These coverings and receptacles may be used to hold, or partially / fully cover, devices such as pacemakers and neurostimulators. The coverings, receptacles and implants described herein, may be made from meshes, webs, lattices, non-wovens, films, fibers, foams, molded, pultruded, machined and 3D printed forms.
Owner:TEPHA INC
Medical devices containing compositions of poly(butylene succinate) and copolymers thereof
PendingUS20210046212A1Reduce usageInhibition of colonizationSuture equipmentsInternal osteosythesisActive agentNonwoven fabric
Resorbable implants, coverings and receptacles comprising poly(butylene succinate) and copolymers thereof have been developed. The implants are preferably sterilized, and contain less than 20 endotoxin units per device as determined by the limulus amebocyte lysate (LAL) assay, and are particularly suitable for use in procedures where prolonged strength retention is necessary, and can include one or more bioactive agents. The implants may be made from fibers and meshes of poly(butylene succinate) and copolymers thereof, or by 3d printing molding, pultrusion or other melt or solvent processing method. The implants, or the fibers preset therein, may be oriented. These coverings and receptacles may be used to hold, or partially / fully cover, devices such as pacemakers and neurostimulators. The coverings, receptacles and implants described herein, may be made from meshes, webs, lattices, non-wovens, films, fibers, foams, molded, pultruded, machined and 3D printed forms.
Owner:TEPHA INC
Detection method for cysteine hydrochloride bacterial endotoxin
The invention discloses a detection method for cysteine hydrochloride bacterial endotoxin. The method comprises the steps of: a. (I) weighing cysteine hydrochloride and sodium carbonate according to a mass ratio of 20:15-20:17 and performing mixing, adding bacterial endotoxin test water to make the concentration of the test article cysteine hydrochloride in the mixed solution at 100mg / ml; (II) diluting the test article cysteine hydrochloride to 1.25mg / ml-5mg / ml with bacterial endotoxin test water; b. taking a dynamic colorimetric method limulus amoebocyte lysate reagent, opening the reagent, then adding bacterial endotoxin test water to perform re-dissolution; and c. mixing 1.25mg / ml-5mg / ml test article cysteine hydrochloride with the re-dissolved limulus amoebocyte lysate reagent in step b at an equal volume, then putting the mixture into a reaction test tube and inserting the test tube into a dynamic test tube detector to calculate the content of endotoxin. The method provided by the invention avoids the disadvantages of animal pyrogen test methods, and has the advantages of strong repeatability, high sensitivity and simple operation.
Owner:CHINA OTSUKA PHARM CO LTD
Anti-interference alkali reagent and preparation method and application method thereof
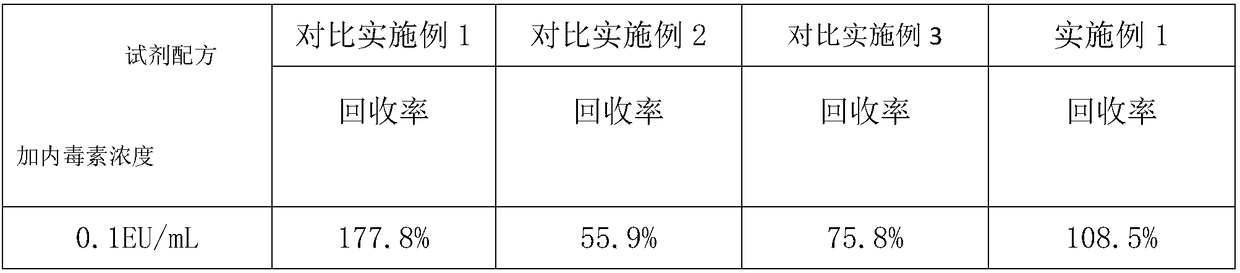
InactiveCN108362542AImprove uniformityImprove stabilityPreparing sample for investigationBiological testingPotassium hydroxideTachypleus
The invention relates to an anti-interference alkali reagent. The anti-interference alkali reagent comprises the following components: potassium hydroxide, sodium chloride, polyprene, bis-glycinate, an ethyleneimine polymer, calcium chloride, magnesium chloride and triton X-100; uniformity and stability of serum can be enhanced by the above components, a lot of protease, blood coagulation factors,and globulin in serum can be simultaneously removed, the protease interference tachypleus amebocyte lysate tests can be greatly reduced, the anti-interference capability is greatly increased, and theideal recovery rate is obtained; an enzyme reaction of a tachypleus amebocyte lysate can be activated at a maximum degree by calcium chloride and magnesium chloride in the formula components, and thereaction capability of endotoxin and the tachypleus amebocyte lysate can be greatly increased; in addition, after usage of the alkali reagent, a Tris-HCl buffer solution is added, the detected serumhas uniformity and stability, is subjected to a cross reaction with endotoxin, an endotoxin structure is protected with a maximum degree, and the endotoxin concentration can be accurately detected.
Owner:FUZHOU XINBEI BIOCHEM IND
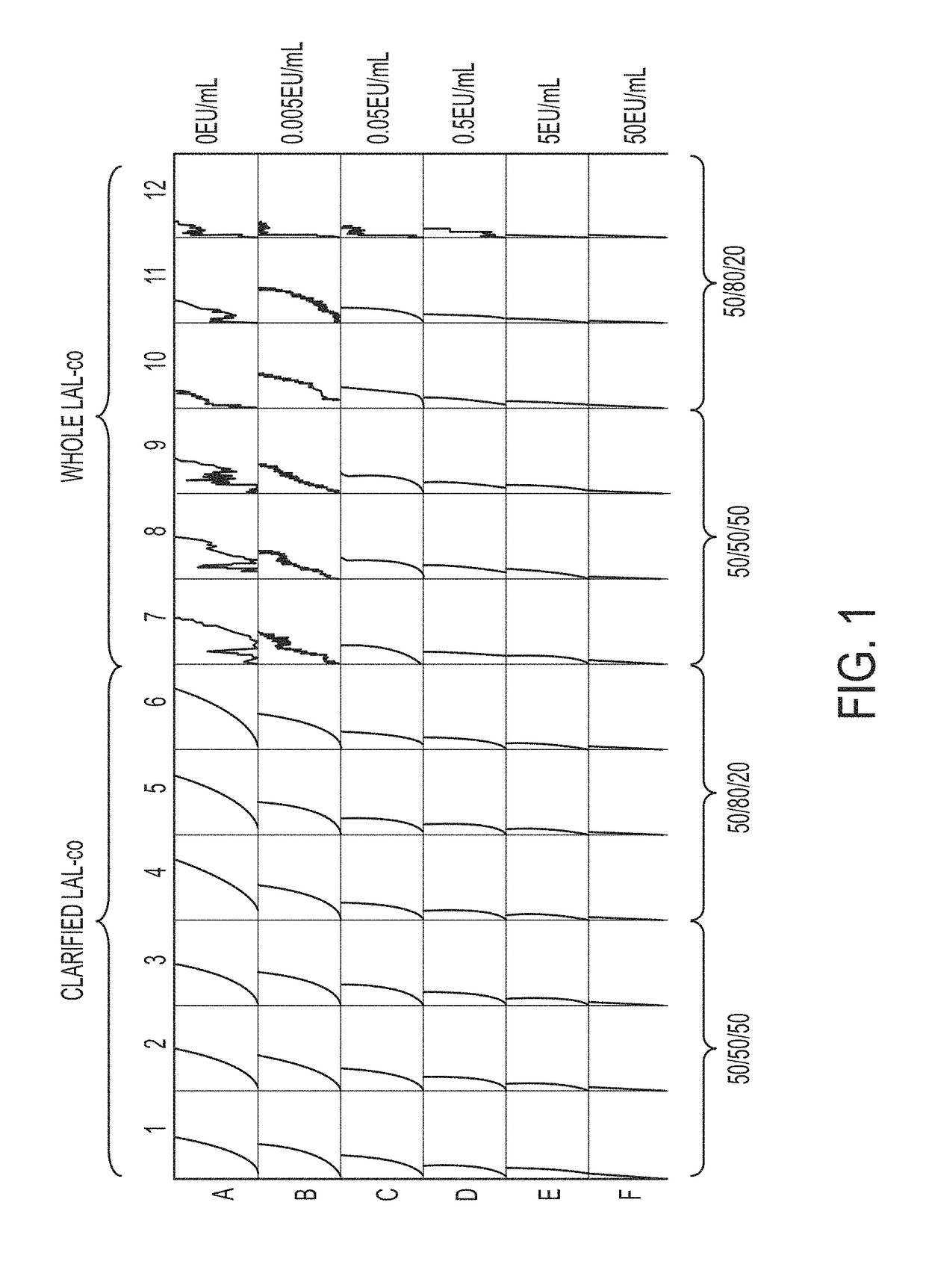
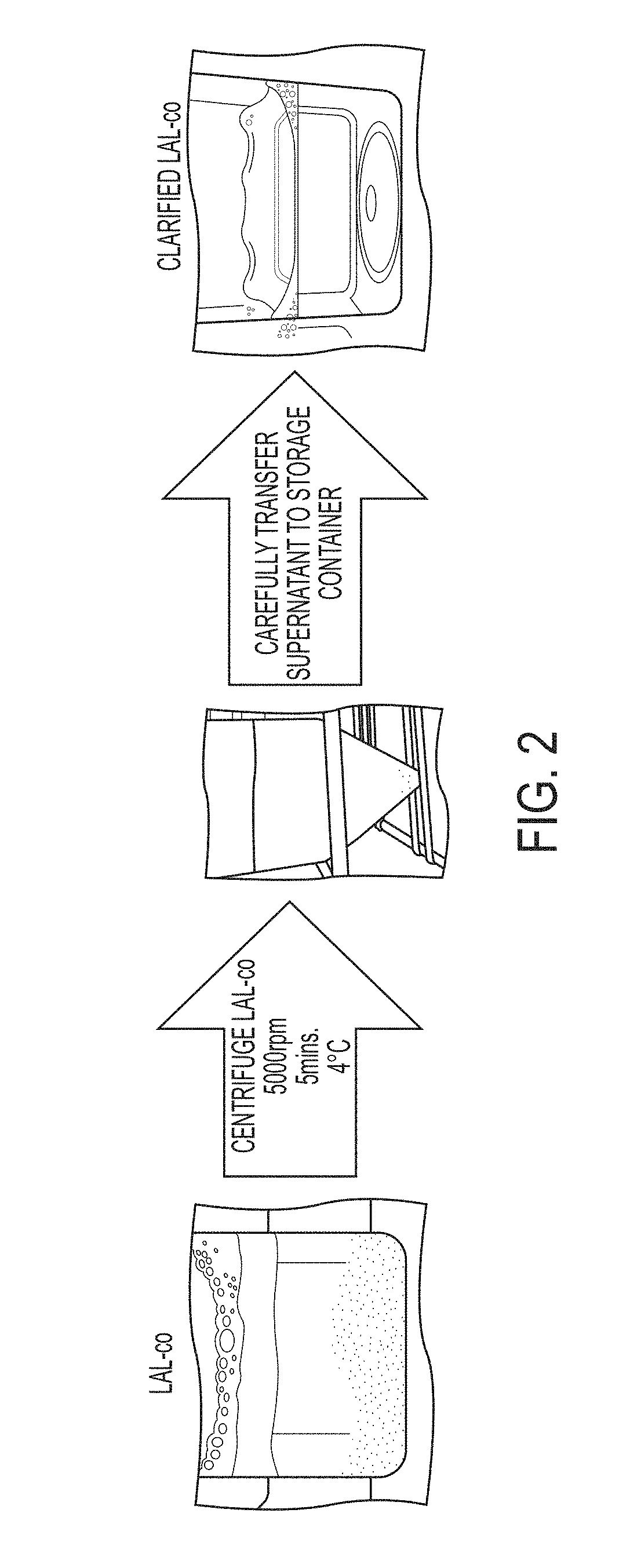
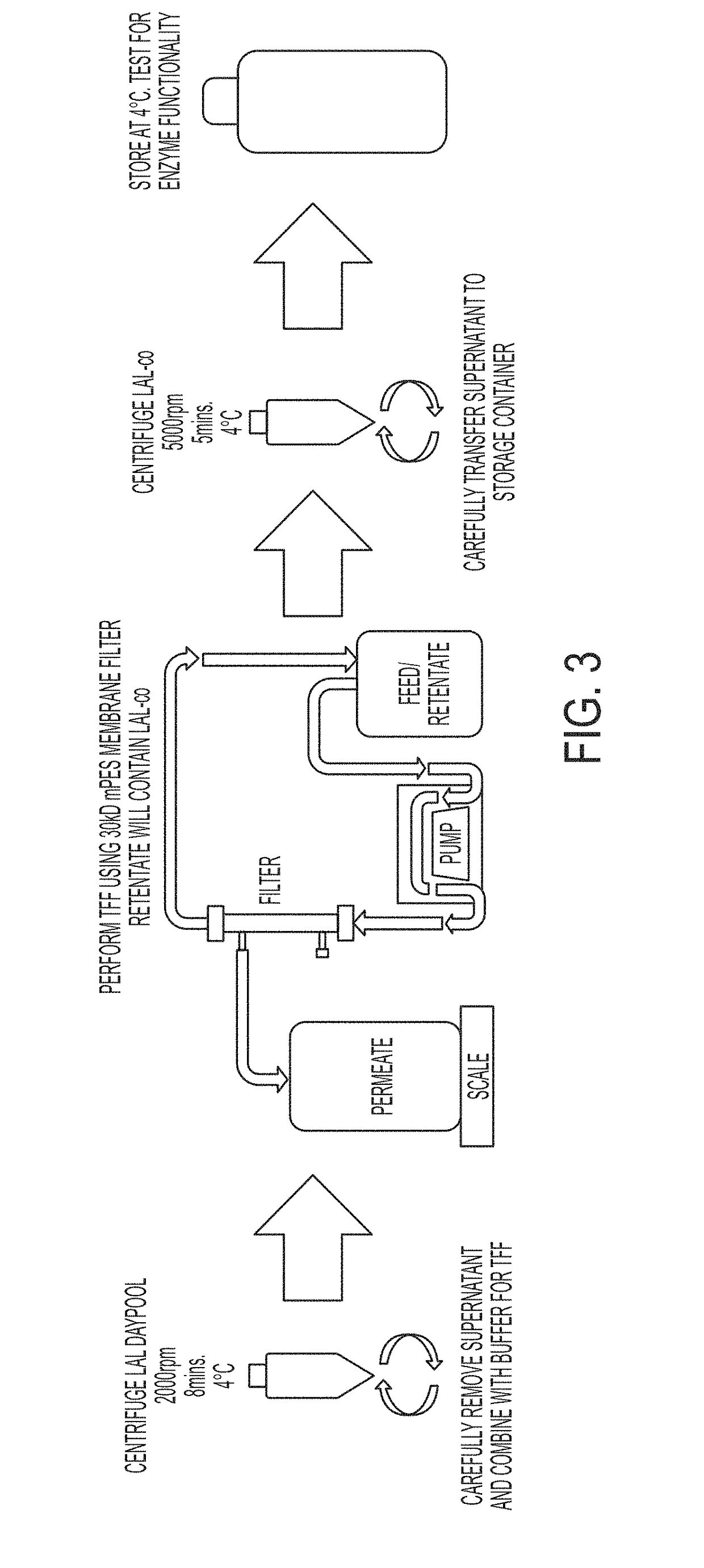
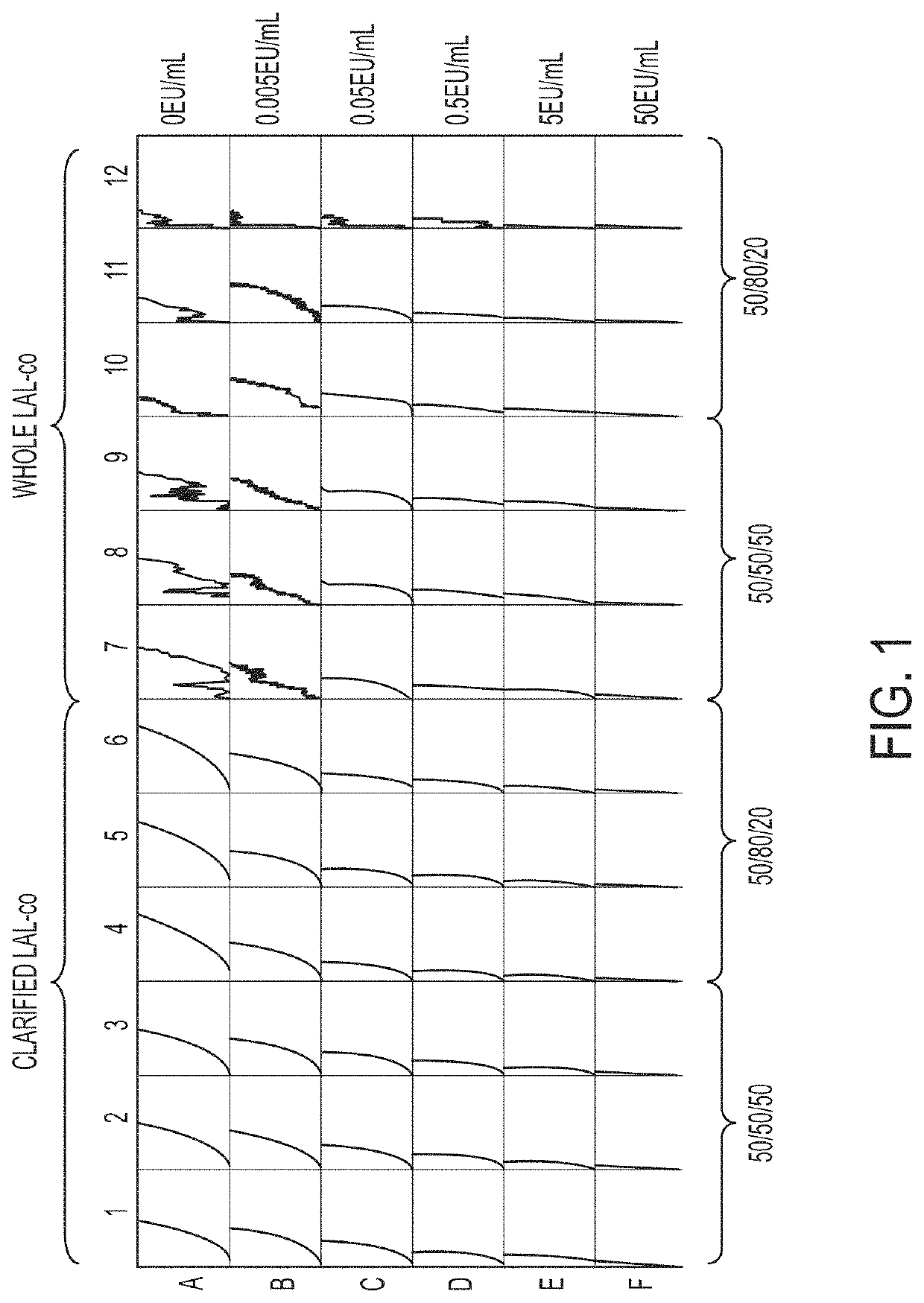
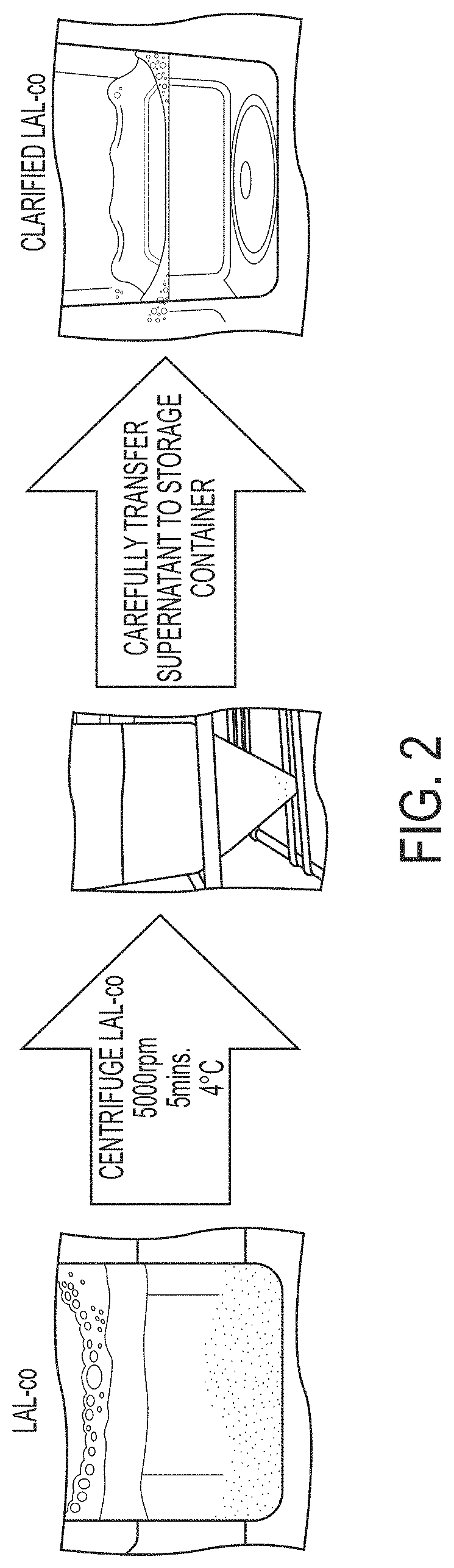
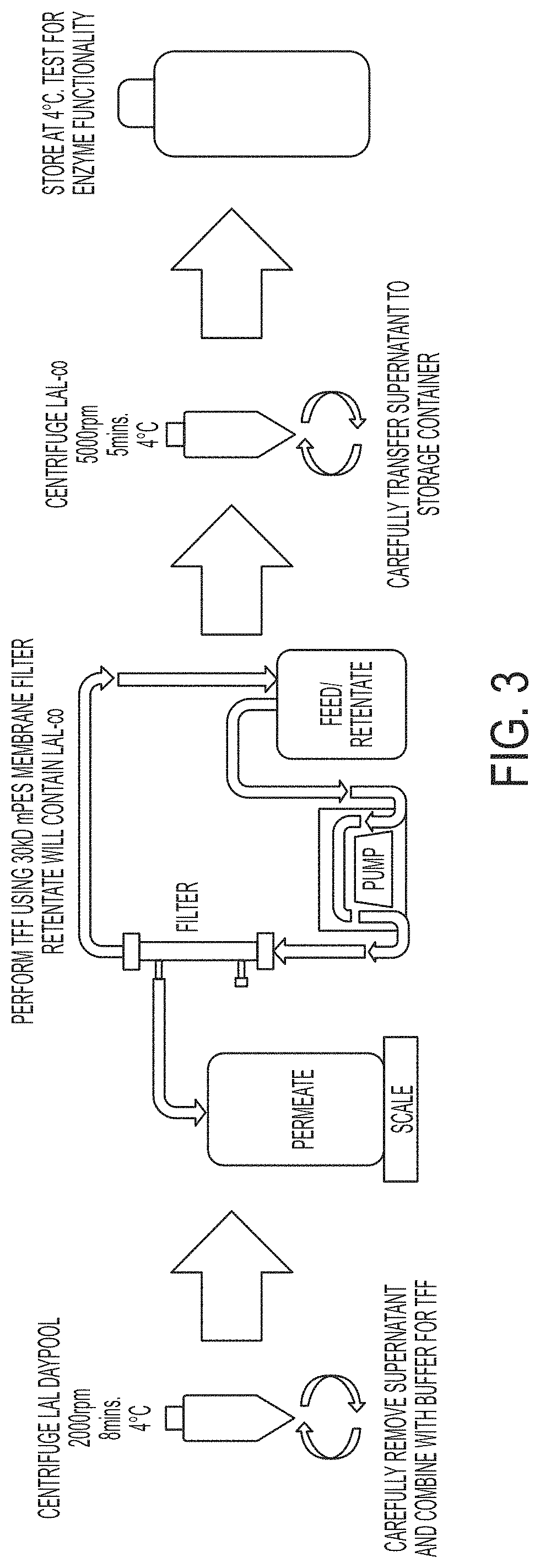
Coagulogen-free clarified limulus amebocyte lysate
ActiveUS20180208964A1High sensitivityMicrobiological testing/measurementBiological material analysisCoagulogenLimulus amebocyte lysate
The present invention is related to compositions comprising clarified limulus amebocyte lysate (LAL), wherein the LAL is substantially free of coagulogen and methods of making such compositions. The invention further relates to a method of detecting an endotoxin in a sample using a chromogenic assay, the method comprising: (a) contacting the sample with a reagent comprising clarified LAL and a chromogenic substrate; and (b) measuring a chromogenic effect resulting from a change in the chromogenic substrate in the presence of endotoxin in the sample, wherein the LAL is substantially free of coagulogen. The invention also relates to kits comprising clarified LAL substantially free of coagulogen, and methods of making such.
Owner:LONZA WALKERSVILLE INC
Method of Detecting an Endotoxin Using Limulus Amebocyte Lysate Substantially Free of Coagulogen
ActiveUS20180038864A1High sensitivityMicrobiological testing/measurementBiological material analysisCoagulogenChromogenic Substrates
The present invention is related to a method of detecting an endotoxin in a sample using a chromogenic assay, the method comprising: (a) contacting the sample with a reagent comprising limulus amebocyte lysate (LAL) and a chromogenic substrate; and (b) measuring a chromogenic effect resulting from a change in the chromogenic substrate in the presence of endotoxin in the sample; wherein the LAL is substantially free of coagulogen. The method also relates to compositions and kits comprising LAL substantially free of coagulogen, and methods of making such.
Owner:LONZA WALKERSVILLE INC
Method for detecting concentration of endotoxin in biochemical tail water by utilizing pressure difference of nanotube membrane
ActiveCN112505279AEliminate the effect of the initial stateImprove accuracyTesting organic contamination in waterNanoparticleVisual inspection
The invention relates to the field of environmental pollution monitoring, in particular to a method for measuring endotoxin in biochemical tail water by utilizing a transmembrane pressure difference of a nanotube membrane. The method comprises the following steps of: preparing a platinum nanotube array with regular arrangement, and carrying out stripping, washing, purifying and substrate processing to prepare a micro membrane assembly; and purifying biochemical tail water by means of an HLB column, filtering the biochemical tail water by using an ultrafiltration membrane, mixing the filtered biochemical tail water with an LAL reagent and continuously injecting the mixed solution into the nanotube membrane assembly, measuring a stable membrane pressure difference in a specified time periodand converting the stable membrane pressure difference into an electric signal, establishing a standard curve, and calculating the endotoxin concentration. According to the method, transmembrane pressure is converted into an electric signal to measure nanoparticles formed by endotoxin and tachypleus amebocyte lysate, so that errors caused by artificial visual inspection are reduced; in addition, the method can reduce the amount of tachypleus amebocyte lysate by more than 20 times, and significantly reduces the single inspection cost and detection time.
Owner:BEIJING NORMAL UNIVERSITY
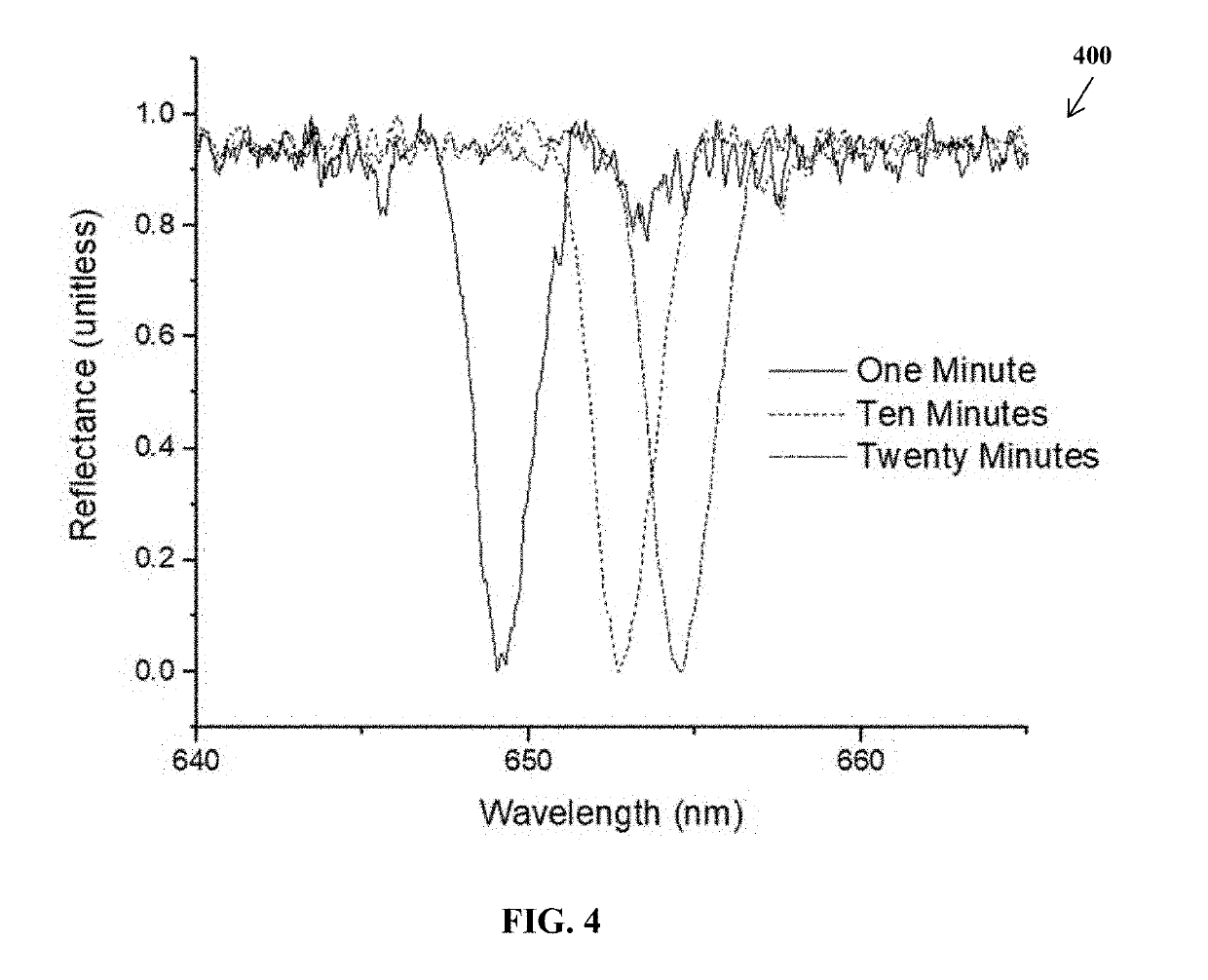
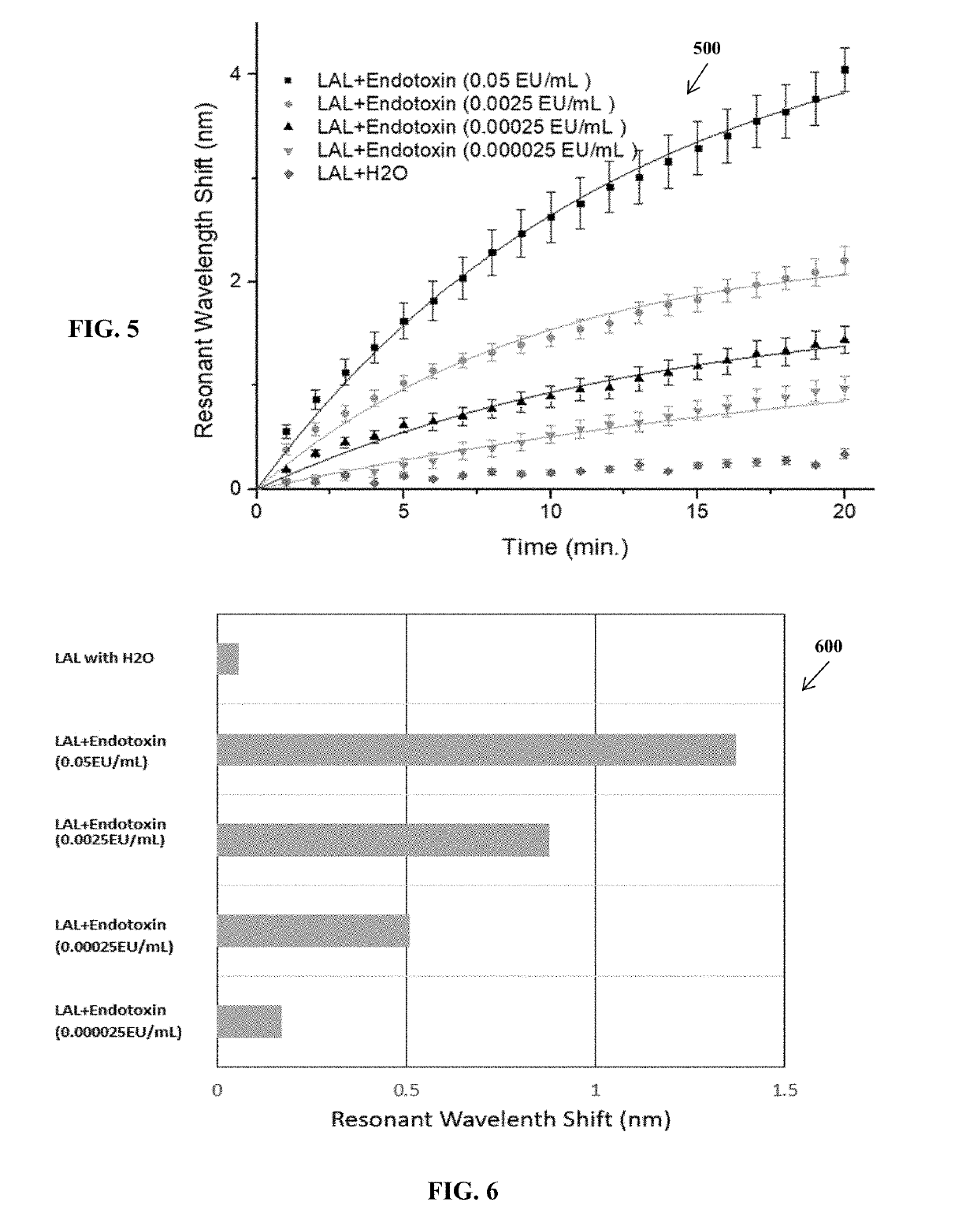
Limulus amoebocyte lysate assay and method of same
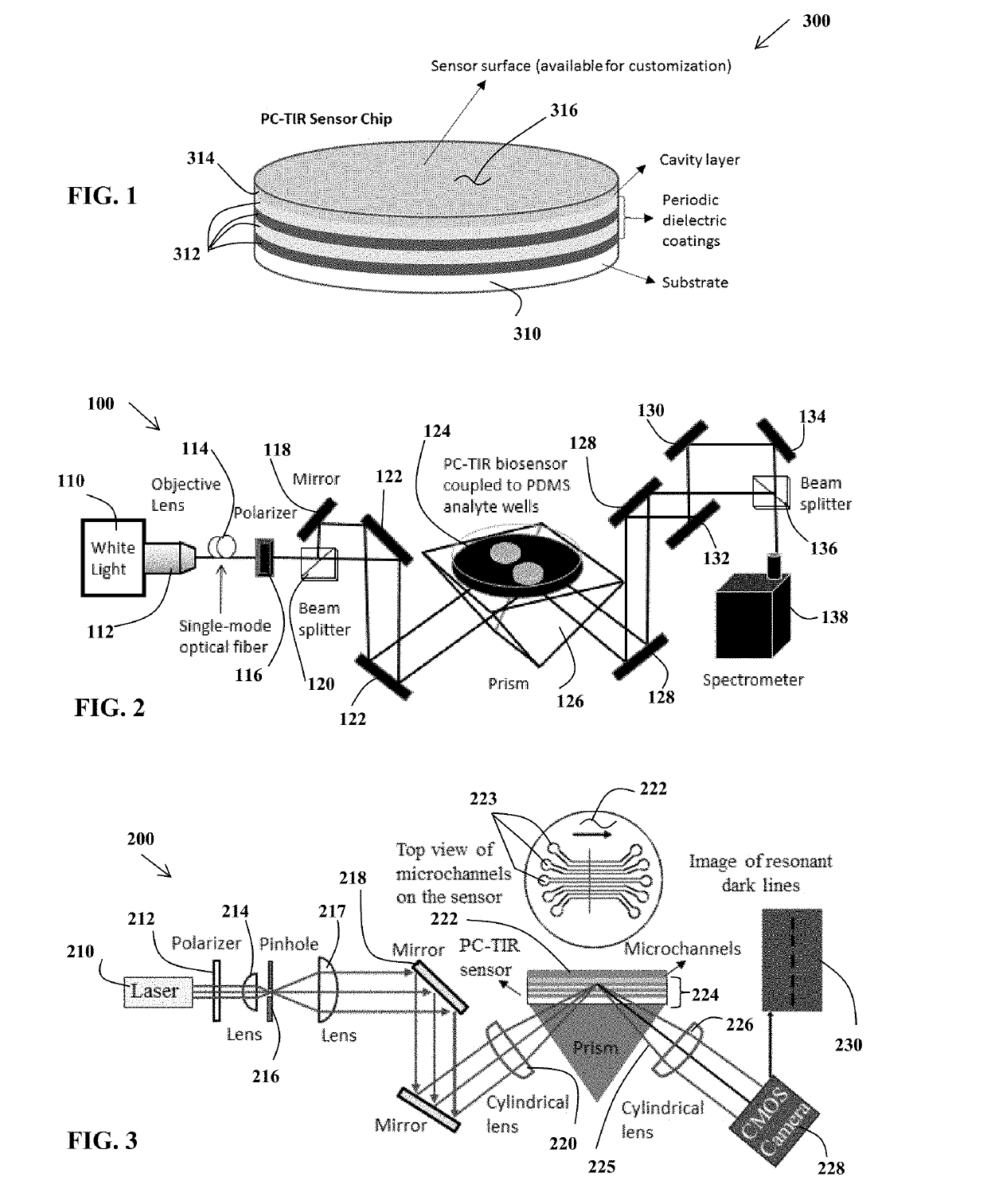
ActiveUS20190113514A1Enhance LAL testingTesting duration has been reducedBiological material analysisColor/spectral properties measurementsRefractive indexWavelength
A pyrogenicity test assay and method of pyrogen testing that allows for rapid and ultrahigh sensitivity testing of parenteral pharmaceuticals or medical devices that contact blood or cerebrospinal fluid by employing a Limulus Amoebocyte Lysate (LAL) assay utilizing a photonic-crystal biosensor. The photonic-crystal biosensor is capable of determining the presence of endotoxins in a test sample by monitoring shifts in the resonant wavelength of an open microcavity affected by the changes in the refractive index of the analyte solutions used.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
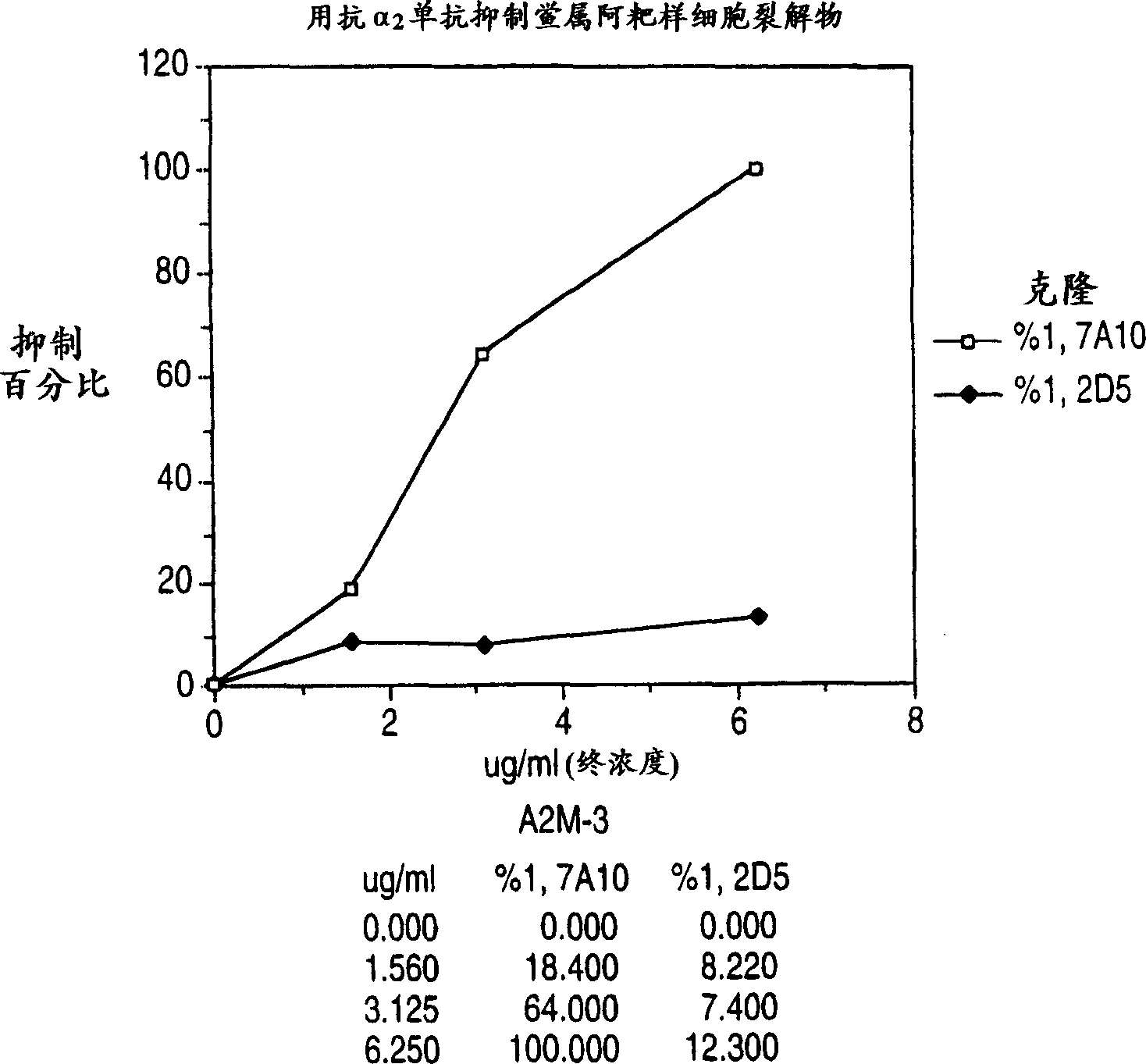
Monoclonal antibody to alpha 2-macroglobulin and its use for detecting glucan
InactiveCN1282339AImmunoglobulins against animals/humansEnzymologyChromogenic SubstratesLimulus amebocyte lysate
The present invention is directed to monoclonal antibodies which specifically bind to a Limulus alpha 2-macroglobulin. Preferred monoclonal antibodies inhibit the enzymatic activation of a Limulus amebocyte lysate to form a gel or to cleave a chromogenic substrate in the presence of an endotoxin but not in the presence of a glucan. The monoclonal antibodies can be used, inter alia, to purify a Limulus alpha 2-macroglobulin, and the preferred antibodies can be used in a method for specifically detecting a glucan in a test sample using an amebocyte lysate.
Owner:BIOWHITTAKER TECH
Improved bacterial endotoxin test for the determination of endotoxins
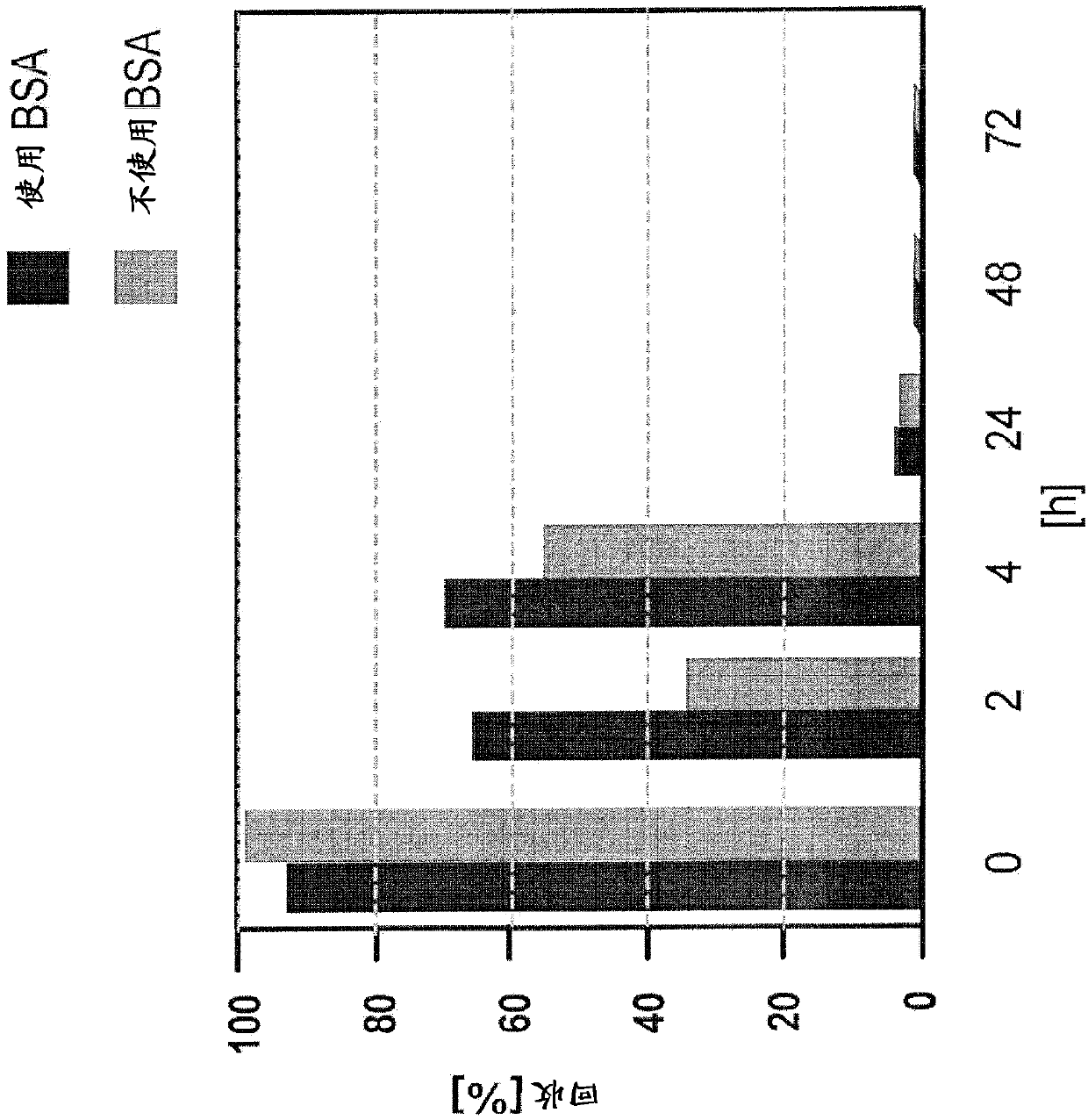
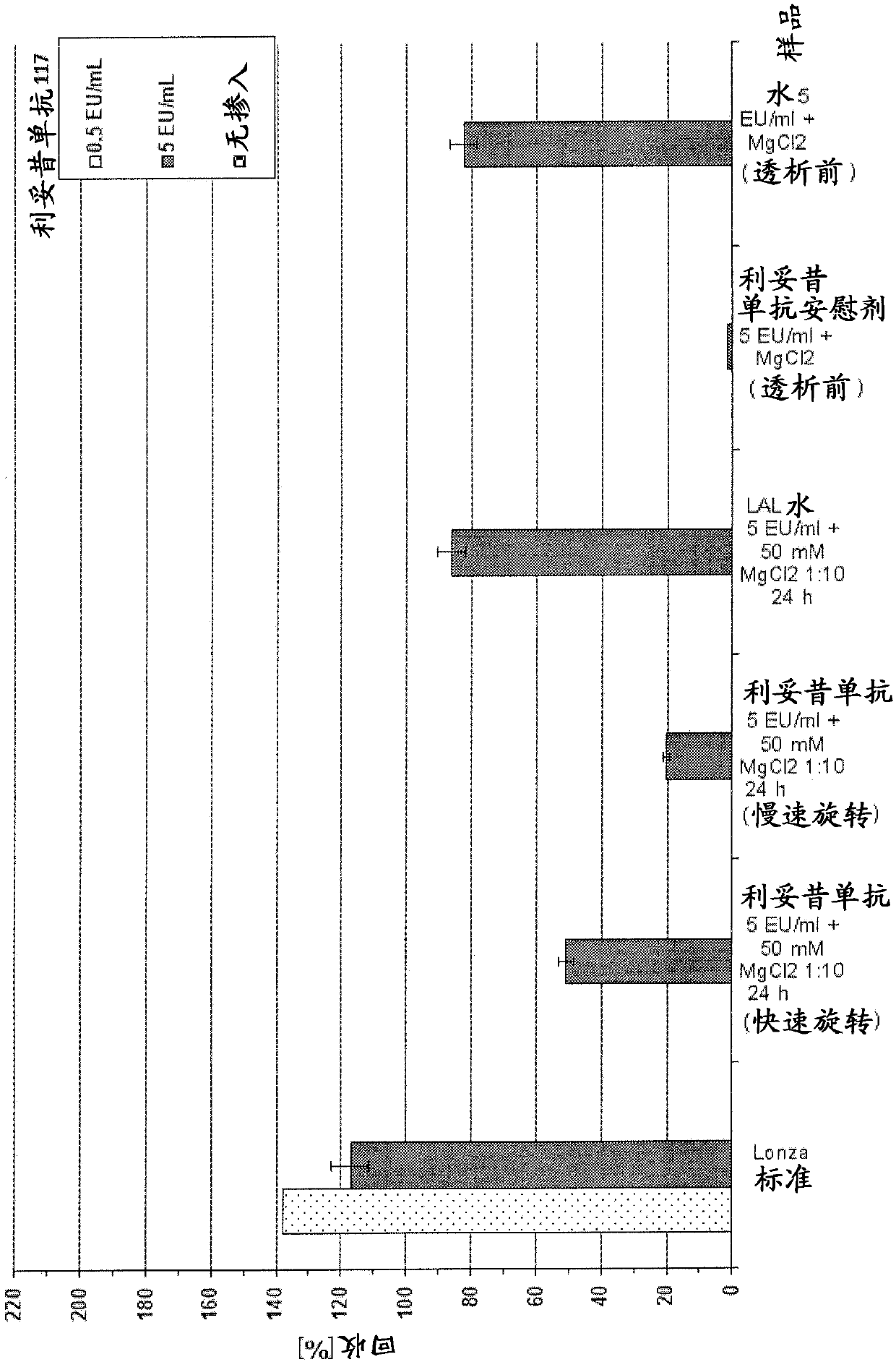
ActiveCN108027374AEasy to detectImprove health statusPreparing sample for investigationBiological material analysisBacteroidesAmoebocyte lysate
Herein is reported a method for determining bacterial endotoxin at low concentrations in a sample of an antibody (that has been produced using bacterial cells) comprising the following steps in the following order: i) adding magnesium ions to the sample, ii) diluting the sample, iii) dialyzing the sample having a pH-value of 5.7-8.0 against an endotoxin-free aqueous solution, and iv) determining bacterial endotoxin in the sample using a bacterial endotoxin test, particularly the limulus amoebocyte lysate assay.
Owner:F HOFFMANN LA ROCHE & CO AG
Peptide combined with endotoxin and application thereof in endotoxin detection
The invention discloses a peptide combined with endotoxin and application of the peptide in endotoxin detection, the peptide at least comprises the following sequence fragments: GCKK YRRF RWKF KGKF WFWG C, and the carboxyl terminal and / or nitrogen terminal of the peptide contains cysteine. The peptide provided by the invention can be used for quantitatively detecting the endotoxin, is low in detection limit, accurate in detection result, high in sensitivity and wide in detection linear range, covers the maximum value of the content of the endotoxin specified in Chinese pharmacopoeia, can be directly used for detecting the endotoxin in water for injection and injection, and does not need to be diluted; the method is high in precision, high in repeatability and short in detection time, and the single detection time of a single sample does not exceed 10 minutes; compared with a limulus reagent, the peptide is convenient to produce and low in cost, is not limited by limulus resources, the quality is stable, and the qualities of different batches have comparability; a prepared biosensor electrode can be repeatedly used for multiple times within a certain time, the result error is small, and the cost can be further reduced.
Owner:GUANGXI BOTANICAL GARDEN OF MEDICINAL PLANTS +1
Detection reagent for specifically detecting (1-3)-beta-D-glucan and preparation method thereof
ActiveCN108866087AHigh sensitivityStrong specificityMicrobiological testing/measurementBiological material analysisZymogenGlucan
The invention belongs to the technical field of gene engineering, and specifically discloses a detection reagent for specifically detecting (1-3)-beta-D-glucan and a preparation method thereof. The nucleotide majorizing sequence of horseshoe crab G factor zymogen alpha subunit, beta subunit and prothrombin gene are obtained respectively and as shown as SEQ ID NO:1-3; the nucleotide sequence for jointly expressing the above three kinds of key factors is further obtained and as shown as SEQ ID NO:4; the eukaryon expression carrier for independently expressing or jointly expressing the three kinds of key factors is successfully constructed, finally an artificial horseshoe crab reagent similar to a natural horseshoe crab reagent is successfully obtained and can be used for detecting (1-3)-beta-D-glucan, the sensitivity is high, the specificity is high, the false positive can be reduced, the preparation technology is simple, proteins do not need to be extracted and purified, the productioncost is low, the demands for wild horseshoe crab sources greatly decreases, and the detection reagent has the wide marketing application prospects.
Owner:GUANGDONG MEDICAL UNIV
Measurement method for physiologically active substance of biological origin, and reagent kit for measurement
InactiveUS20130052657A1Easy to prepareImprove accuracyMaterial analysis by observing effect on chemical indicatorBiological testingDrugs solutionMicroparticle
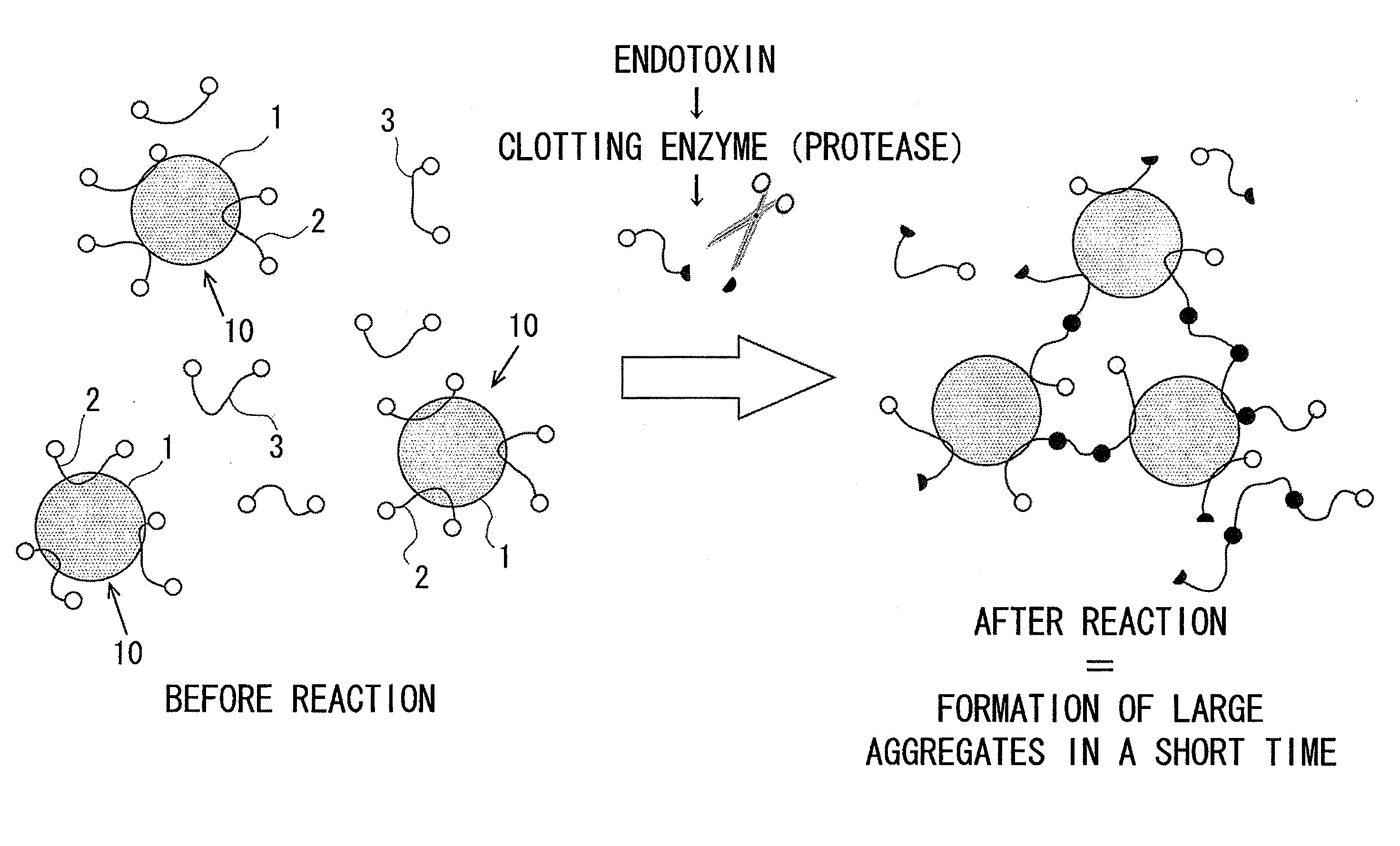
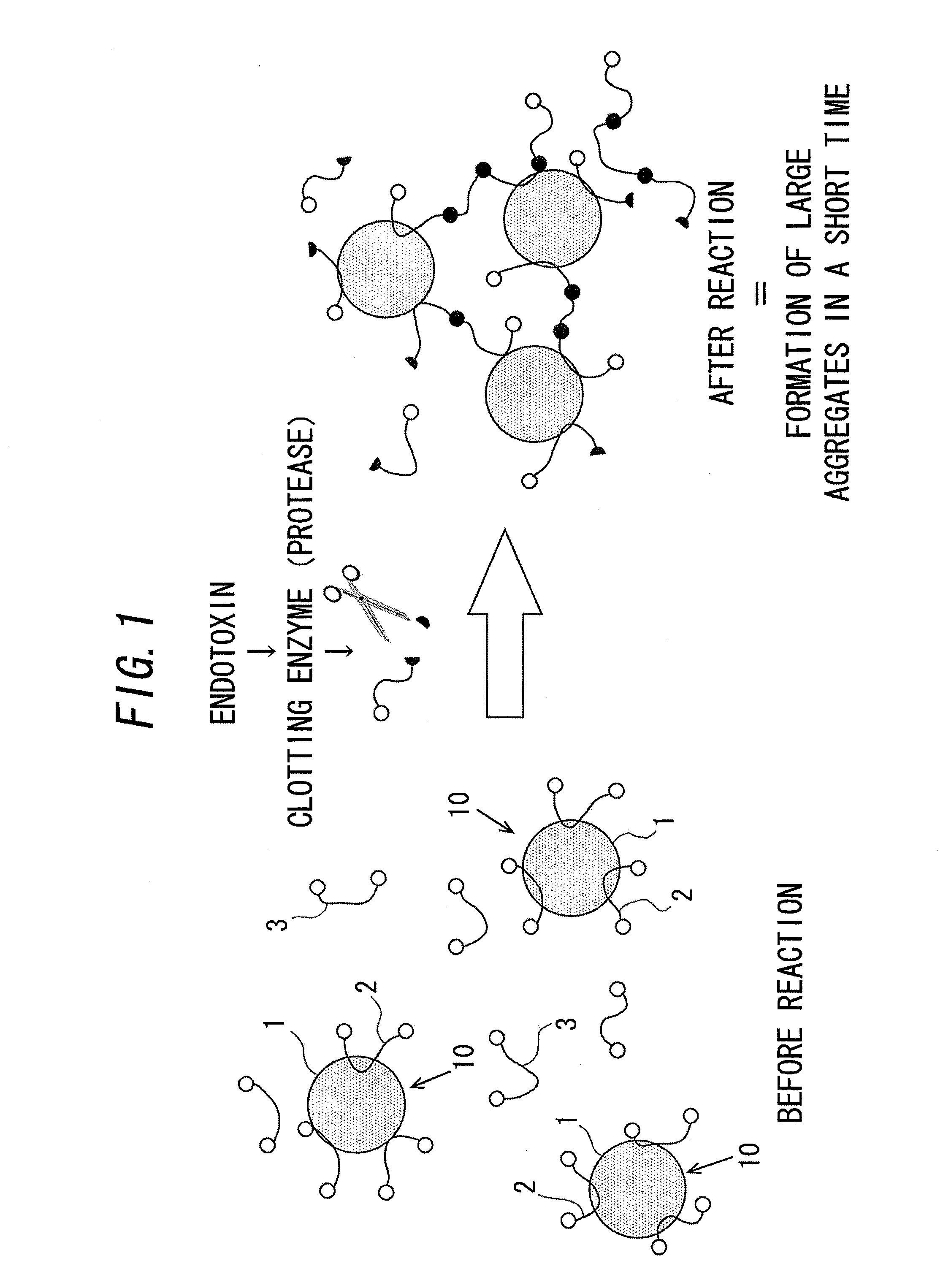
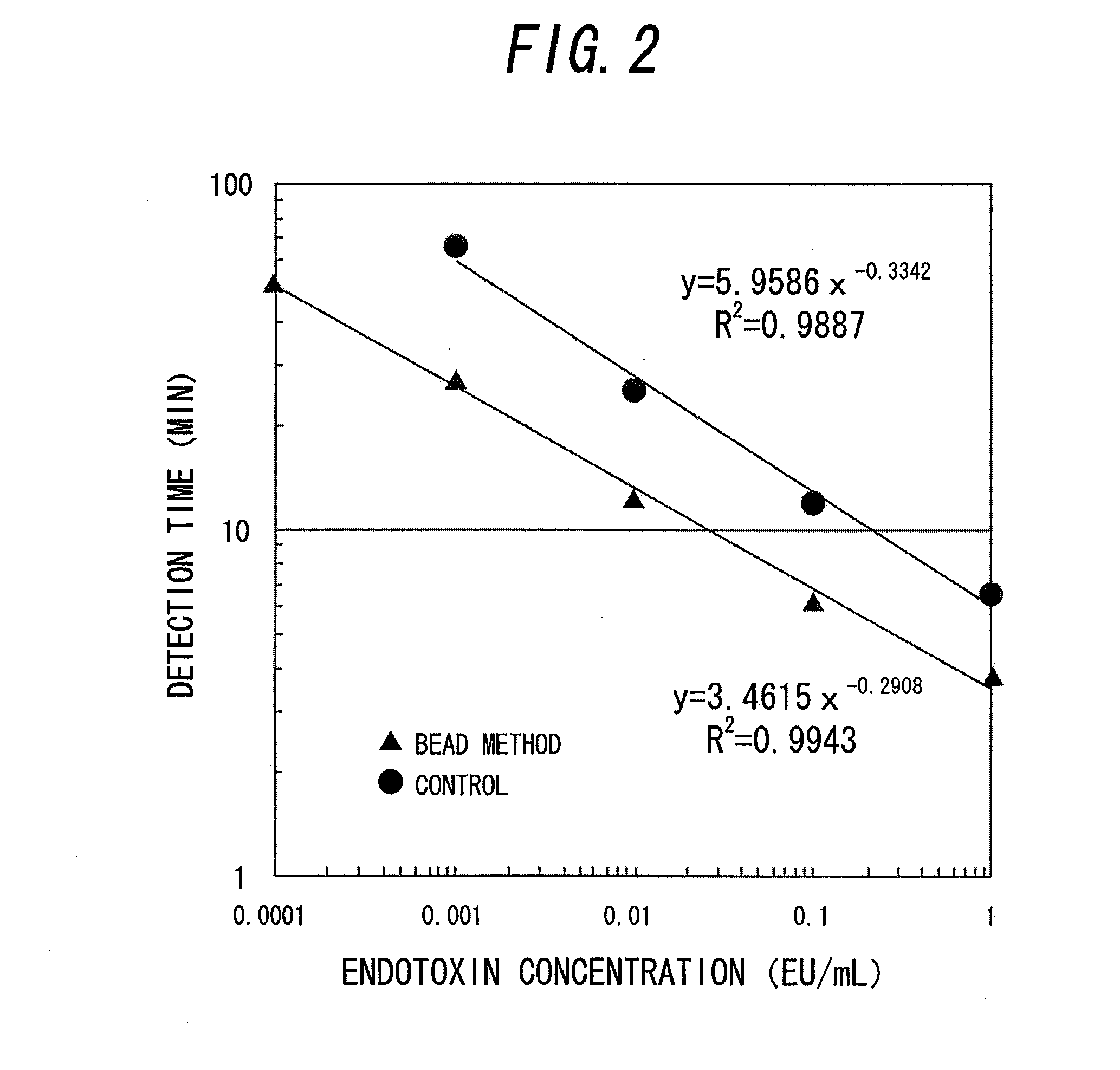
Disclosed is a technique which simplifies the adjustment of a reagent which makes the proteins contained in limulus amoebocyte lysate (LAL) attach to the surface of microparticles dispersed in a prepared drug solution, and which can improve the accuracy of the detection of a predetermined physiologically active substance or the measurement of the concentration thereof. A reagent is prepared which adsorbs the proteins contained in LAL to beads dispersed in a drug solution prepared in advance. By reacting a sample containing an endotoxin with the reagent, groups of the beads associate and rapidly form large aggregates, and measurement of the endotoxin is carried out by the detecting the formation of the aggregates. The beads are formed from an inorganic material such as alumina, kaolin, or manganese oxide.
Owner:KOWA CO LTD
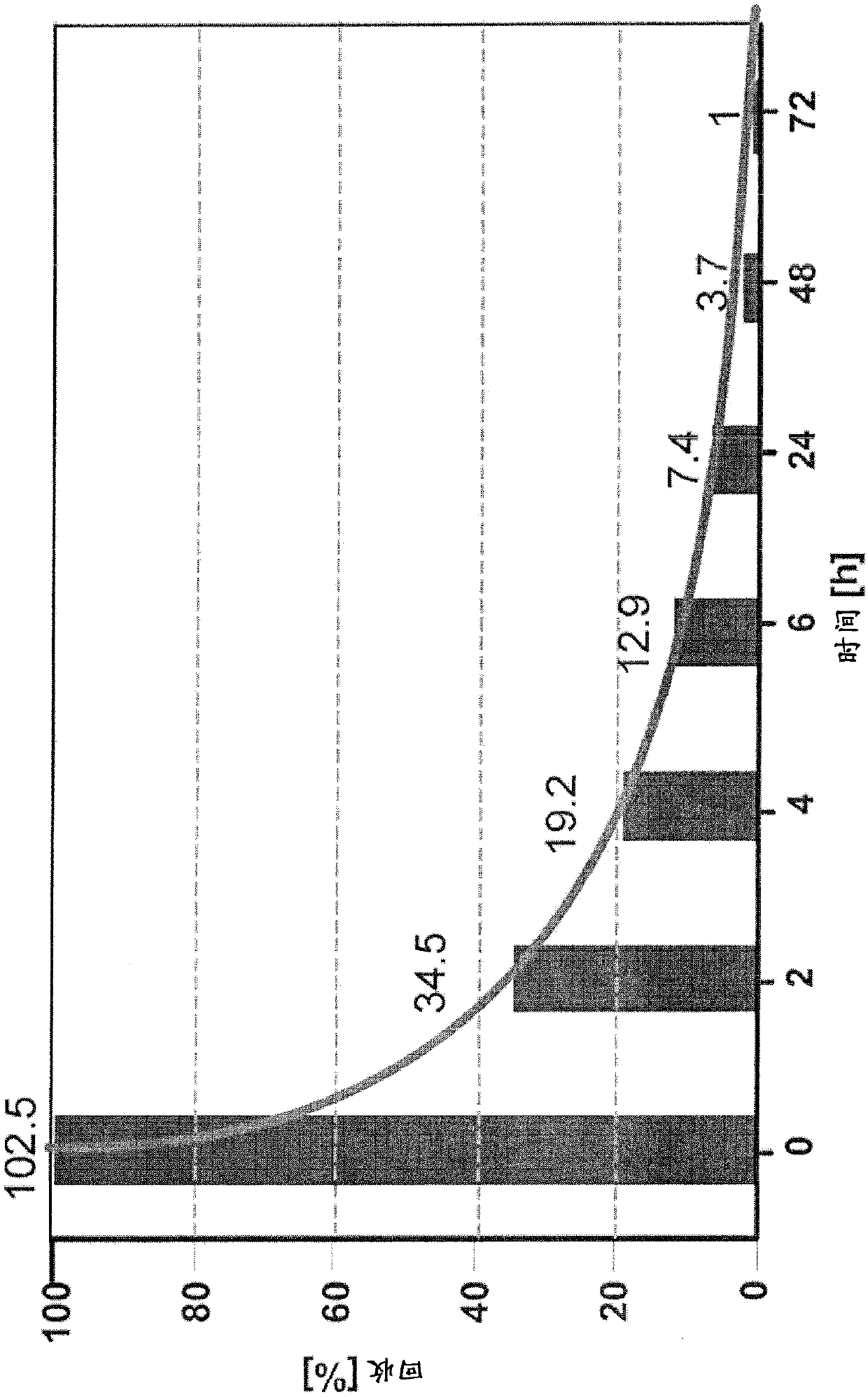
Lyophilized tachypleus amebocyte lyophilized microspheres as well as preparation method and application thereof
PendingCN113092767ADo not interfere with detection functionFast formingMaterial analysisAgainst vector-borne diseasesMicrosphereActive agent
The invention relates to lyophilized tachypleus amebocyte lysate microspheres as well as a preparation method and application thereof. The lyophilized tachypleus amebocyte lyophilized microspheres comprise amebic cell extracts of tachypleus amebocyte lyophilized microspheres and an active agent, wherein the active agent comprises polyvinylpyrrolidone, lactose, mannitol, raffinose, polyethylene glycol and glycine. All the components cooperate with one another to promote the formation of the tachypleus amebocyte lysate, improve hardness of the formed tachypleus amebocyte lysate and protect activity of the tachypleus amebocyte lysate in the precooling period, the freeze-drying period and the storage period, so the tachypleus amebocyte lysate freeze-dried microspheres are high in hardness, smooth in surface, not prone to breaking and chipping, high in stability and convenient for subsequent automatic or manual sub-packaging and storage.
Owner:DYNAMIKER BIOTECH TIANJIN
Coagulogen-free clarified limulus amebocyte lysate and chromogenic assay of endotoxin
ActiveUS11352656B2High sensitivityMicrobiological testing/measurementBiological material analysisCoagulogenLimulus amebocyte lysate
Owner:LONZA WALKERSVILLE INC
Yarns and fibers of poly(butylene succinate) and copolymers thereof, and methods of use thereof
PendingUS20220202988A1Improve retentionReduce usageSuture equipmentsMammary implantsYarnActive agent
Resorbable implants comprising poly(butylene succinate) and copolymers thereof have been developed. The implants are preferably sterilized, and contain less than 20 endotoxin units per device as determined by the limulus amebocyte lysate (LAL) assay, and are particularly suitable for use in procedures where prolonged strength retention is necessary, and can include one or more bioactive agents. The implants may be made from fibers and meshes of poly(butylene succinate) and copolymers thereof, or by 3d printing, and the fibers may be oriented. Coverings and receptacles made from forms of poly(butylene succinate) and copolymers thereof have also been developed for use with cardiac rhythm management devices and other implantable devices. These coverings and receptacles may be used to hold, or partially / fully cover, devices such as pacemakers and neurostimulators. The coverings and receptacles are made from meshes, webs, lattices, non-wovens, films, fibers, and foams, and contain antibiotics such as rifampin and minocycline.
Owner:TEPHA INC
Coagulogen-free clarified limulus amebocyte lysate
ActiveUS20220267824A1High sensitivityMicrobiological testing/measurementBiological material analysisCoagulogenLimulus amebocyte lysate
The present invention is related to compositions comprising clarified limulus amebocyte lysate (LAL), wherein the LAL is substantially free of coagulogen and methods of making such compositions. The invention further relates to a method of detecting an endotoxin in a sample using a chromogenic assay, the method comprising: (a) contacting the sample with a reagent comprising clarified LAL and a chromogenic substrate; and (b) measuring a chromogenic effect resulting from a change in the chromogenic substrate in the presence of endotoxin in the sample, wherein the LAL is substantially free of coagulogen. The invention also relates to kits comprising clarified LAL substantially free of coagulogen, and methods of making such.
Owner:LONZA WALKERSVILLE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com