Improved bacterial endotoxin test for the determination of endotoxins
A bacterial endotoxin and endotoxin technology, applied in the preparation of test samples, measuring devices, and resistance to vector-borne diseases, etc., can solve the problems of underestimation and undetection of endotoxin pollution, so as to improve health status and improve Detection, life-saving effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0269] Example 1: Technical Equipment and Reagents
[0270] 1. Technical equipment
[0271] 1.1 Microplate reading system (also referred to as "reader" herein)
[0272] · PRO, Multimode Microplate Reader; Tecan / Tecan Deutschland GmbH, Switzerland, P / N: 30050303, Germany.
[0273] ·Magellan V.7.1 software
[0274] ·Costar TM Cell culture plate, 96-well, Fisher Scientific, P / N: 07-200-89.
[0275] 1.2 Shaker system and glass vials
[0276] • Multi Reax; Heidolph, Germany, P / N: 545-10000-00.
[0277] • 1.5 ml screw top glass bottle (N8); Macherey-Nagel GmbH & Co. KG, Germany, P / N: 702004 (Qty. 100).
[0278] · N 8PP nut, black, top closed; Macherey-Nagel GmbH & Co. KG, Germany, P / N: 70250 (Qty. 100).
[0279] • 4 ml screw top glass bottle (N13); Macherey-Nagel GmbH & Co. KG, Germany, P / N: 702962 (Qty. 100).
[0280] · N 13PP nut, black, top closed; Macherey-Nagel GmbH & Co. KG, Germany, P / N: 702051 (Qty. 100).
[0281] 1.3 Dialysis equipment
[0282] ·Rotary Dialyz...
Embodiment 2
[0318] Embodiment 2: The present invention overcomes the method for LER effect
Embodiment 21
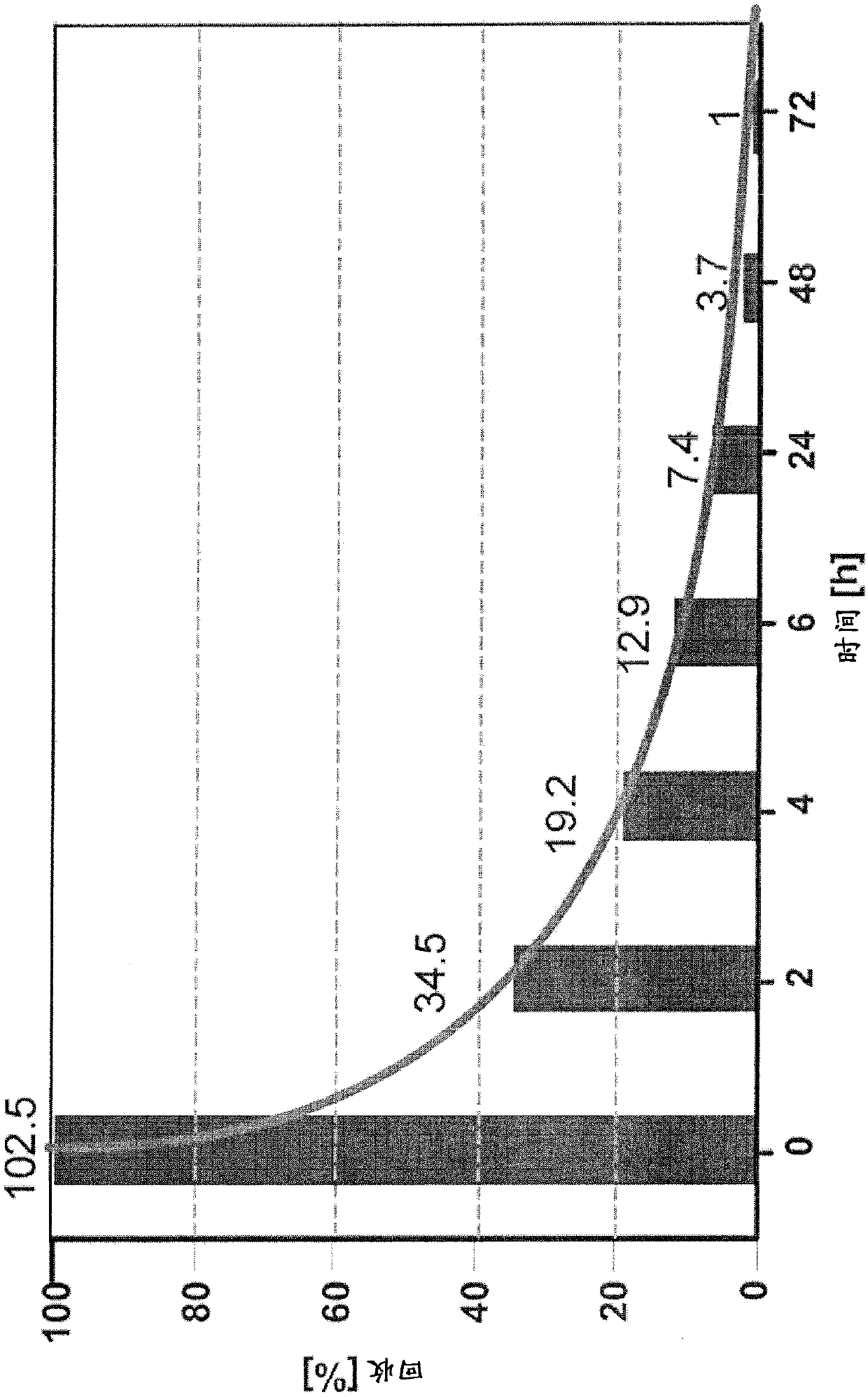
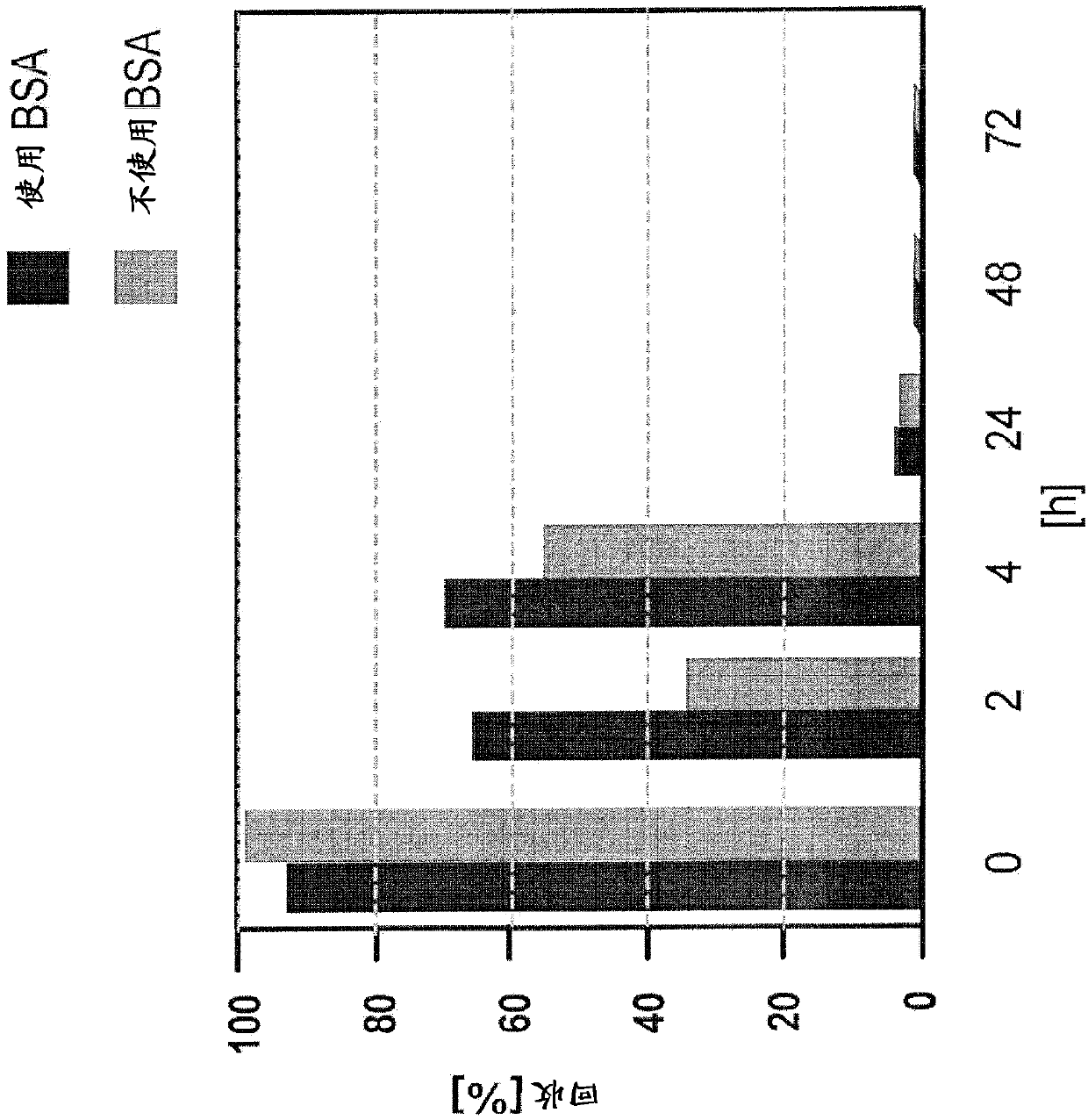
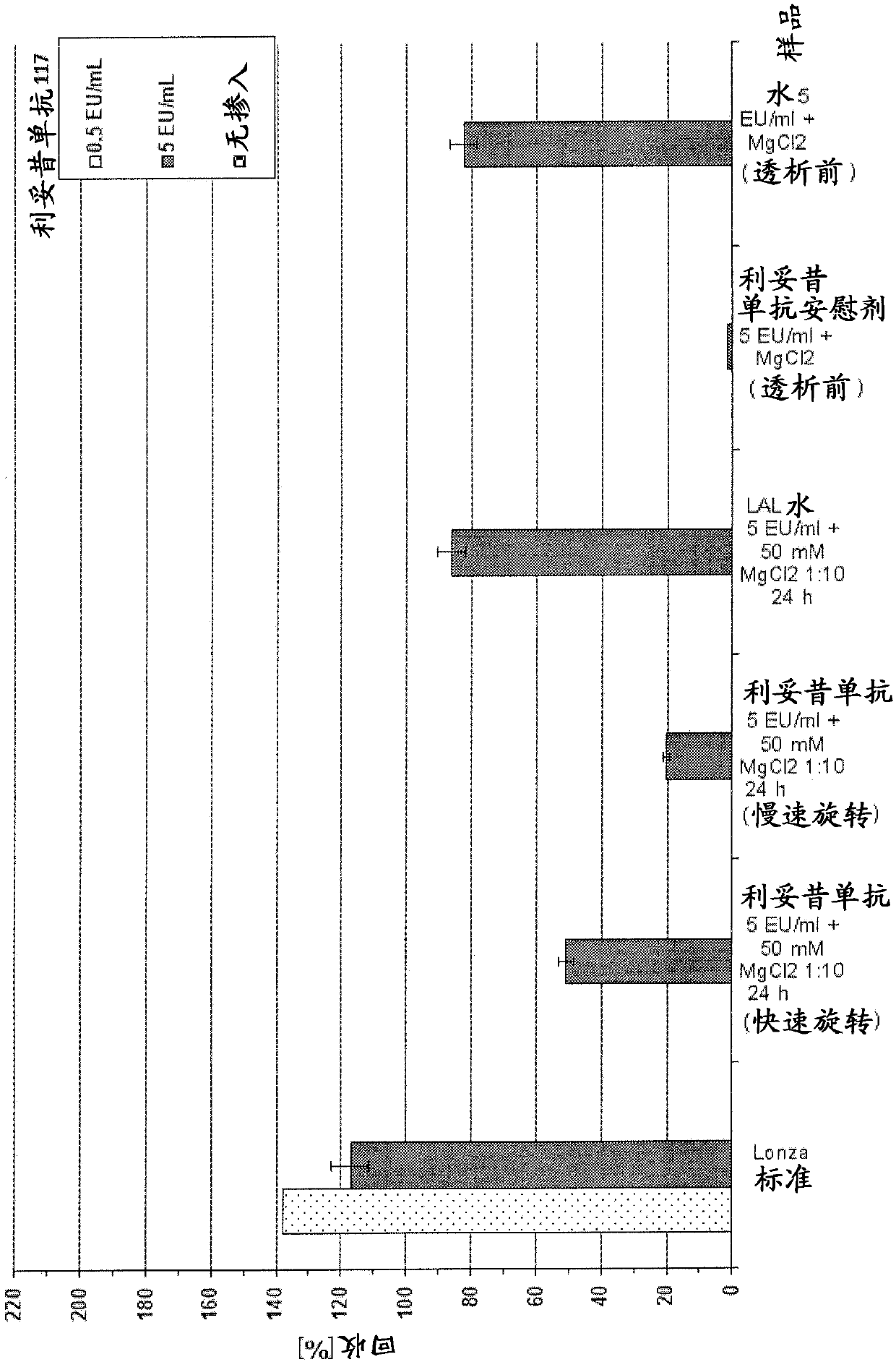
[0319] Example 2.1: A solution to overcome the LER effect
[0320] In this example, rituximab and rituximab placebo were used as samples. However, as described below, the protocols described herein can be used to overcome LER in all drug antibody typical formulations.
[0321] Materials used in this example
[0322] -membrane:
[0323] 10kDa cellulose acetate (CA) membrane from Harvard Apparatus, USA, P / N: SP1 7425-CA10K
[0324] - Dialyzer:
[0325] FastSpin DIALYZER, chamber volume 1000 μl, Harvard Apparatus, USA, P / N 740510 (Qty.1) or 740504 (Qty.5)
[0326] - Sample vials:
[0327] ·1.5ml screw top glass bottle (N8); Macherey-Nagel GmbH&Co.KG, Germany, P / N: 702004
[0328] · N 8PP nut, black, top closed; Macherey-Nagel GmbH&Co.KG, Germany, P / N: 70250
[0329] - Crystallization dish:
[0330] ·900ml, Duran, VWR Germany, P / N:216-1817
[0331] -MgCl 2 - stock solution:
[0332] · 1M MgCl2 (magnesium chloride hexahydrate) in water for analysis ACS, ISO, Reag. P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com