Monoclonal antibody to alpha 2-macroglobulin and its use for detecting glucan
A monoclonal antibody, macroglobulin technology, applied in the field of glucan detection, can solve the problem of not being able to develop analytical glucan and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
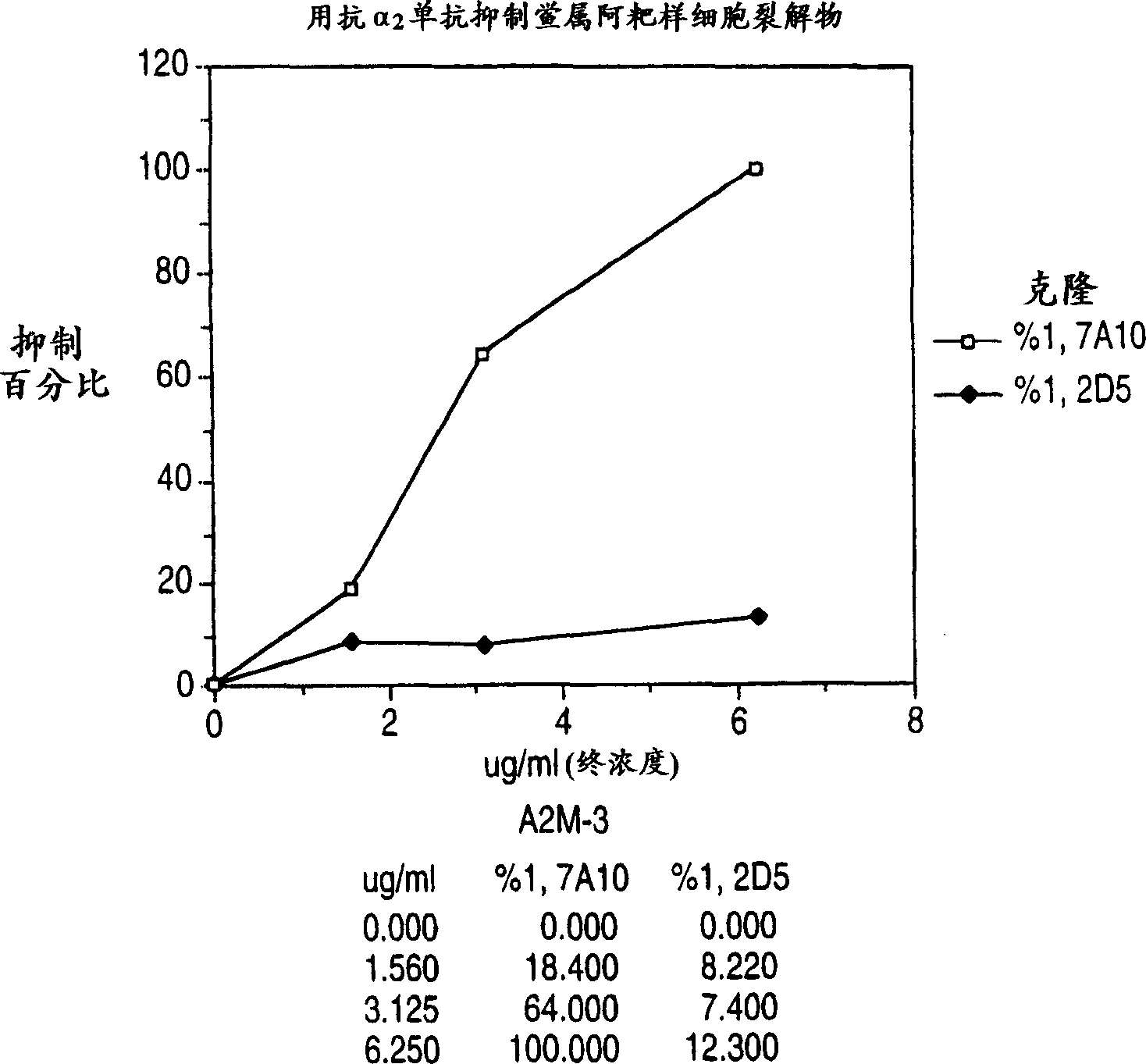
[0041] This example demonstrates the inhibition of the response of amebocyte lysates to endotoxin but not dextran in the presence of monoclonal antibody 7A10.
[0042] The monoclonal antibody 7A10 was added to the Limulus amebocyte lysate to a final concentration of 1.560 μg / ml, 3, 125 μg / ml, and 6.250 μg / ml. The percentage inhibition of the endotoxin response by the lysate was assessed by measuring the inhibition of the cleavage of the chromogenic substrate in the control lysate without mAb and in the lysate samples spiked with different concentrations of mAb. Each lysate sample was challenged by adding 0.5 Eu / ml endotoxin.
[0043] The response of the lysate sample to this level of endotoxin was completely inhibited when the concentration of mAb 7A10 was 6.250 μg / ml ( figure 1 ). Elevated concentrations of a second mAb, 2D5, which also interacts with Limulus α 2 - specifically binds to one epitope on macroglobulin.
[0044] Addition of purified monoclonal antibody 7A10 c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com