Method for detecting bacterial endotoxin in Shenmai injection
A technology for bacterial endotoxin and shenmai injection, applied in the field of medicine, can solve the problems of inability to monitor quality, cumbersome and time-consuming operation, etc., and achieve the effects of reducing inspection cost, fast method, and improving inspection efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
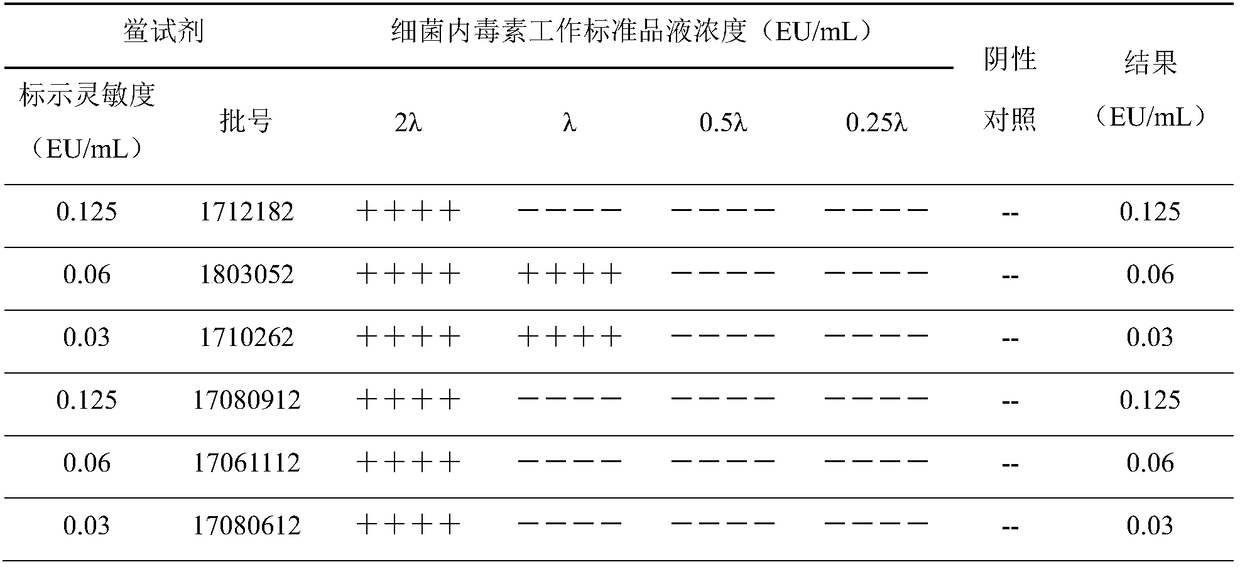
[0018] 1. Experimental materials and instruments
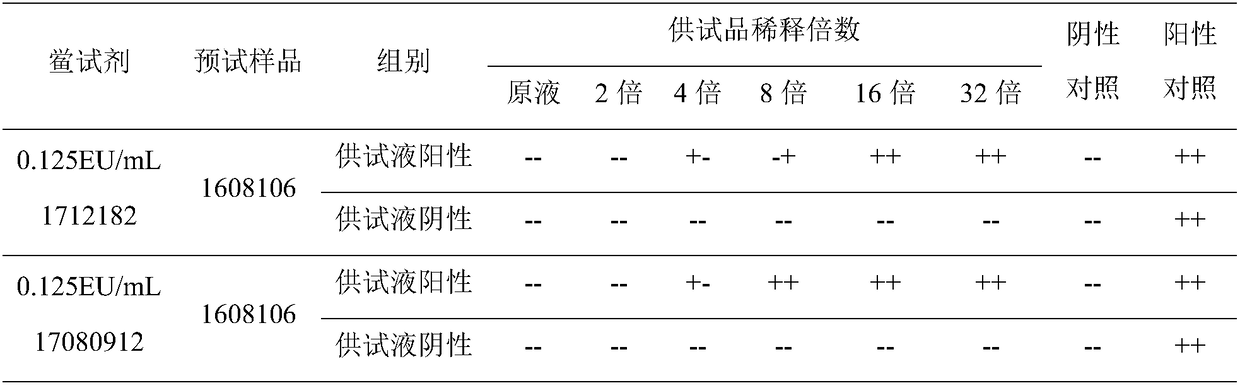
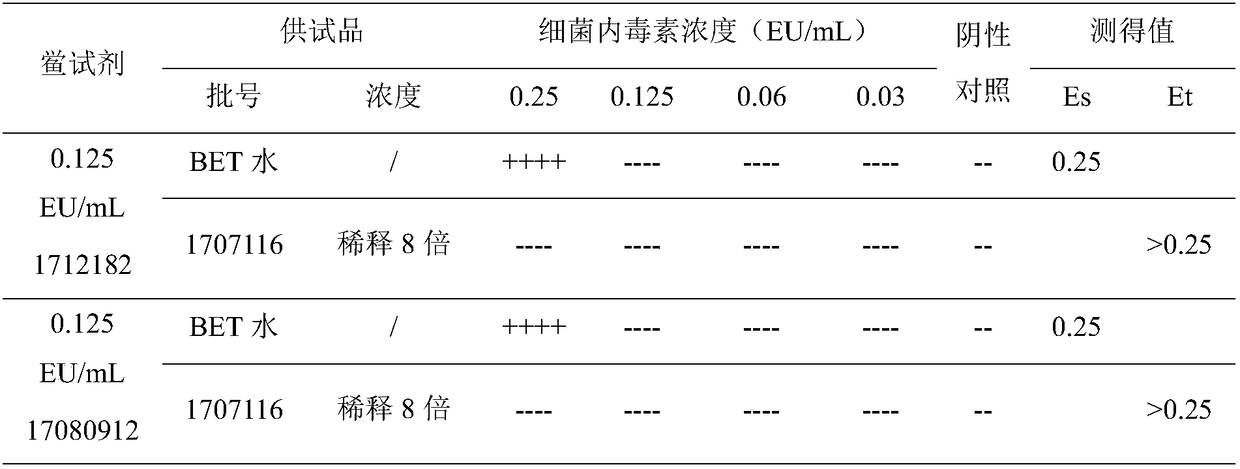
[0020] Labeled sensitivity (λ) 0.125EU / mL, specification 0.1mL / bottle, batch number 1712182; labeled sensitivity (λ) 0.06EU / mL, specification 0.1mL / box, batch number 1803052; labeled sensitivity (λ) 0.03EU / mL, specification 0.1 mL / tube, batch number 1710262; valid for two years, produced by Zhanjiang Andus Biological Co., Ltd.
[0021] Labeled sensitivity (λ) 0.125EU / mL, specification 0.1mL / batch, batch number 17080912; labeled sensitivity (λ) 0.06EU / mL, specification 0.1mL / batch, batch number 17061112; labeled sensitivity (λ) 0.03EU / mL, specification 0.1 mL / tube, batch number 17080612; valid for two years, produced by Fuzhou Xinbei Biochemical Industry Co., Ltd.
[0022] 1.2 Bacterial endotoxin inspection water
[0023] Specification 100mL / bottle, batch number 1710260, containing bacterial endotoxin <0.003EU / mL, valid for three years, produced by Zhanjiang Andus Biological Co., Ltd.
[0024] 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com