Method for checking bacterial endotoxin in Shenxiong glucose injection
A technology of ginseng glucose and bacterial endotoxin, which is applied in the field of bacterial endotoxin inspection, can solve the problems of cumbersome and time-consuming operation, cannot use quality monitoring, etc., and achieves the effects of reducing inspection cost, facilitating production process monitoring and inspection work, and detecting accurate detection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0016] 1. Experimental materials and instruments
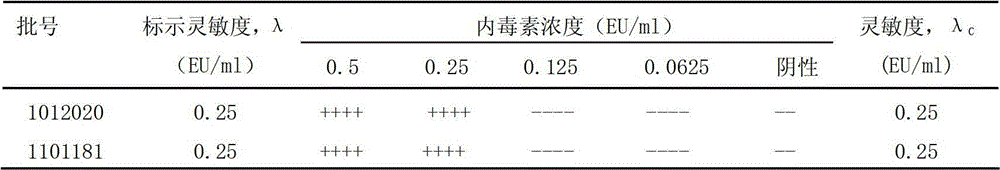
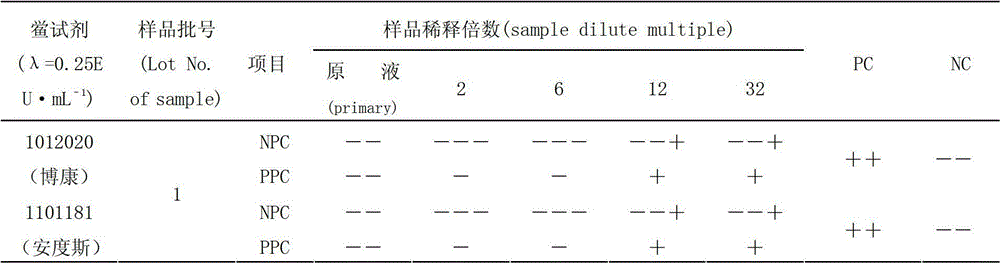
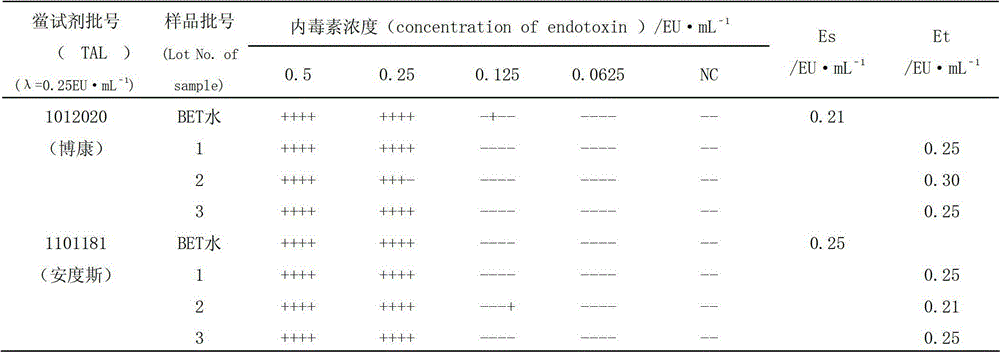
[0017] Limulus reagent (TAL): Zhanjiang Bokang Marine Biological Co., Ltd., specification: 0.1mL, batch number: 1012020, sensitivity: 0.25EU·mL -1 ; Zhanjiang Andus Biological Co., Ltd., specification: 0.1mL, batch number: 1101181, sensitivity: 0.25EU·mL -1 .
[0018] Bacterial endotoxin working standard (WSE): China Institute for the Control of Pharmaceutical and Biological Products, potency: 120EU / bottle, batch number: 150601-201072.
[0019] Bacterial endotoxin test water (BET): China Institute for the Control of Pharmaceutical and Biological Products, specification: 10mL, batch number: 2008-04.
[0020] Shenxiong Glucose Injection: Guizhou Jingfeng Injection Co., Ltd., specification 100mL, batch number: 1, 2, 3.
[0021] Instruments: electric drying oven, ET-96 bacterial endotoxin gel tester, vortex mixer, micropipette, pyrogen-free tip, test tube, test tube rack, 75% alcohol cotton ball, parafilm, scissors, grinding wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com