Method for detecting fleroxacin bacterial endotoxin used for injection
A technology of bacterial endotoxin and fleroxacin, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems that samples cannot be accurately detected and interference cannot be eliminated, and achieve the effect of improving quality standards, easy operation, and eliminating interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] 1. Experimental materials:
[0012] 1.1 Test product: fleroxacin for injection
[0013] Production unit: Shenyang Xinma Pharmaceutical Co., Ltd.
[0014] Specification: 0.1g Batch number: 20100703, 20100704, 20100705;
[0015] Specification: 0.2g Batch number: 20101221, 20101222, 20101223;
[0017]
[0018] 1.3 Bacterial endotoxin working standard (CSE)
[0019] Production unit: China Institute for the Control of Pharmaceutical and Biological Products, batch number 150601-201065, potency 180 EU / bottle.
[0020] 1.4 Bacterial endotoxin test water (BET water)
[0021] Production unit: Zhanjiang Andus Biological Co., Ltd., specification: 5ml, batch number: 1002050.
[0022] 1.5 Special diluent for blood preservation solution
[0023] Production unit: Zhanjiang Andus Biological Co., Ltd., specification: 5ml, batch number 1008310.
[0024] 2. Determination of limit value
[0025] Clinical usage and dosage of this product: 0.2-0.4g (ca...
Embodiment 2
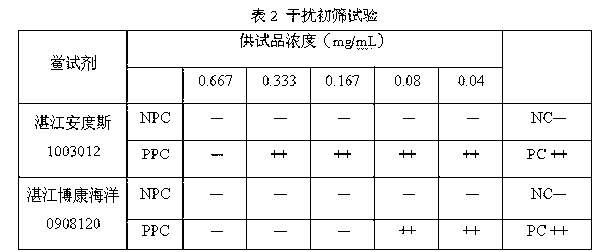
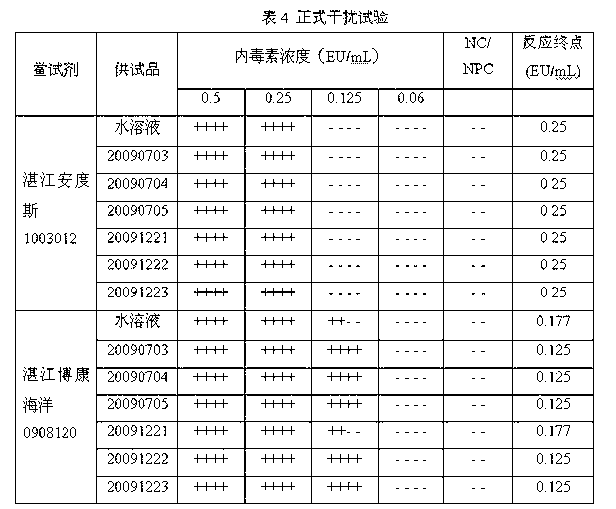
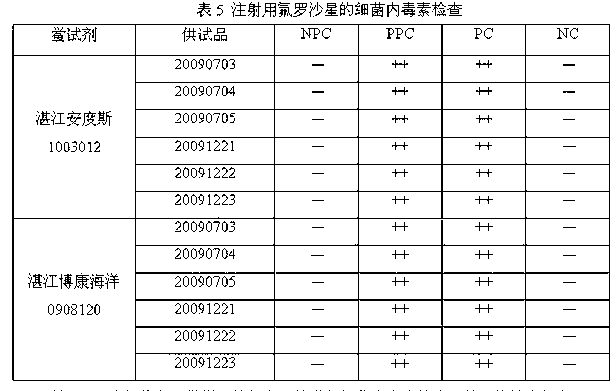
[0055] Dilute the bacterial endotoxin working standard to 0.25 EU / ml with bacterial endotoxin test water; dilute fleroxacin for injection to 0.667 mg / ml with a special diluent for blood preservation solution to make NPC, and use 1.333 mg / ml for the test solution Dilute it with 0.50 EU / ml endotoxin solution to make PPC. Results: NPC and NC were negative reactions, PPC and PC were positive reactions, and the bacterial endotoxin test results met the requirements.
Embodiment 3
[0057] Dilute the bacterial endotoxin working standard to 0.5EU / ml with bacterial endotoxin test water; dilute fleroxacin for injection with a special diluent for blood preservation solution to a 20mg / ml solution, and then prepare the dilution with bacterial endotoxin test water To 0.08mg / ml to make NPC, use 0.16mg / ml test solution and 1.0 EU / ml endotoxin solution to dilute in equal amount to make PPC. Results: NPC and NC were negative reactions, PPC and PC were positive reactions, and the bacterial endotoxin test results met the requirements.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com