Method for measuring bacterial endotoxin content in injection grade granulesten by developing substrate method
A technology of bacterial endotoxin and soybean phospholipids, which is applied in the measurement of color/spectral characteristics and the preparation of test samples, etc., to achieve the effect of reducing detection costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1. Determination of Bacterial Endotoxin Limit (L)
[0025] Referring to the import registration standard, the endotoxin content of lecithin for injection should be ≤6EU / g, that is, 0.006EU / mg.
Embodiment 2
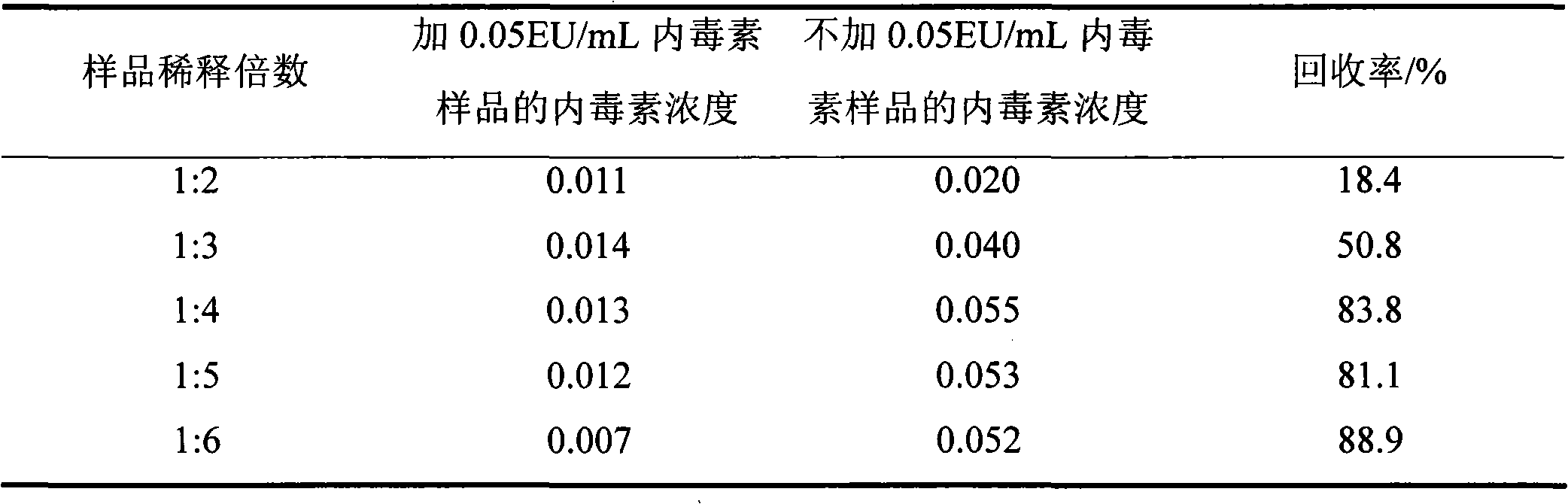
[0026] Embodiment 2. the determination of the preparation of need testing solution and maximum dilutable factor (MVD)
[0027] The sample was accurately weighed under sterile conditions, and the phospholipids were dissolved into an initial solution of 10 mg / mL with bacterial endotoxin test water (BET water) (the concentration of the initial solution of 1 mg / mL was too small and exceeded the detection limit; the initial solution of 100 mg / mL A standard curve with a higher concentration is required, but the solution is turbid and the interference is enhanced). The limit L is 0.006EU / mg, the lowest concentration of the standard curve is 0.01EU / mL, and the maximum dilution factor of the test sample
Embodiment 3
[0028] Embodiment 3. standard curve reliability test
[0029] The establishment of the standard curve was carried out according to the operation steps of bacterial endotoxin inspection. Dilute the 0.1EU / mL endotoxin solution into a 0.01-0.1EU / mL bacterial endotoxin standard solution. Draw a standard curve with the absorbance value as the ordinate and the endotoxin concentration as the abscissa. The concentration is regressed with the absorbance value, and the regression equation is y=24.514x-0.0242, R 2 =0.9908, |r|≥0.980, so the standard curve is established.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com