Plasma diluent kit for bacterial endotoxin gel process detection of blood plasma and use method thereof
A technology of bacterial endotoxin and diluent, which is applied in the direction of biological testing, material inspection products, etc., can solve the problems of complicated operation of turbidity method, expensive equipment, protein influence, etc., achieve low cost, improve work quality and work efficiency, and operate simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
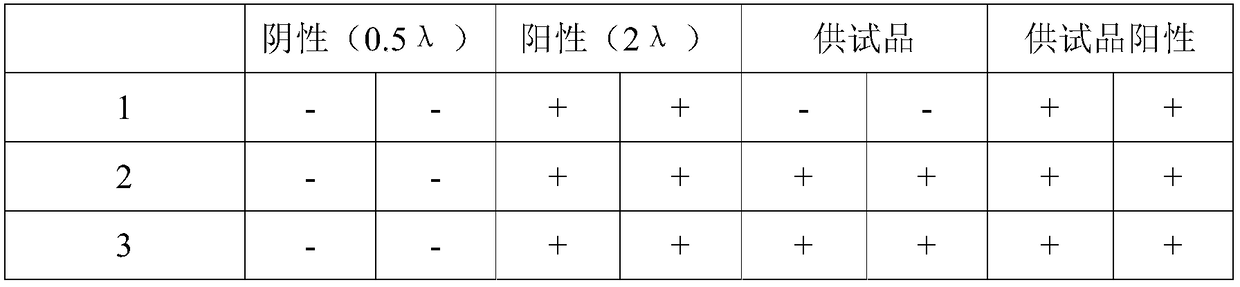
[0010] Specific embodiment one: In this embodiment, a blood plasma diluent kit for detection of plasma by bacterial endotoxin gel method comprises diluent A, diluent B and diluent C; wherein said diluent A has a concentration of 0.1-0.3mg / mL protamine sulfate solution; Diluent B is CaCl with a concentration of 8.0-10.0mg / mL 2 Solution; dilution C consists of Hepes and Triton X-100, the final concentration of Hepes in dilution C is 8-10mmol / L, and the final concentration of Triton X-100 is 0.3%-0.5%.
[0011] The beneficial effect of this implementation mode:
[0012] This embodiment adopts different reagents for anti-interference dilution according to the different action principles of different anticoagulants, which is highly targeted, effective, and the dilution factor is small, only 8 times of dilution is enough. The whole experiment operation is simple, without adding other auxiliary equipment , no need to change the experimental equipment and equipment, the cost is low, ...
specific Embodiment approach 2
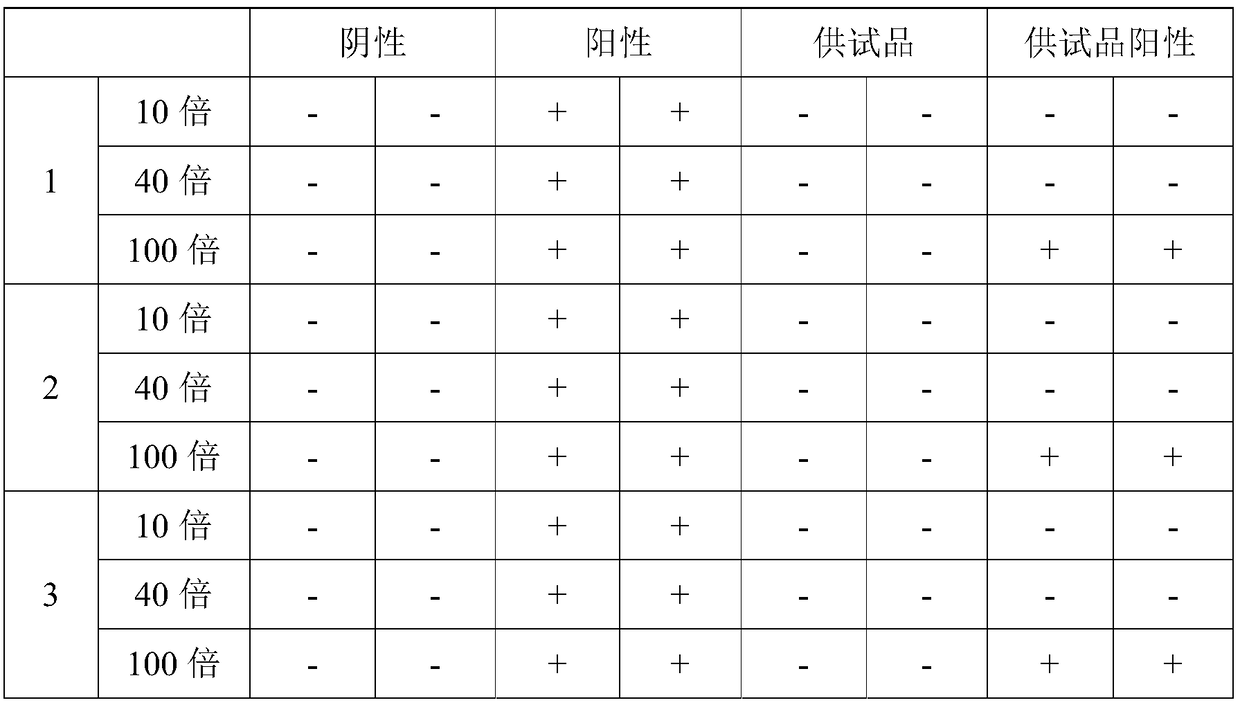
[0013] Embodiment 2: This embodiment differs from Embodiment 1 in that the diluent A is a protamine sulfate solution with a concentration of 0.2 mg / mL. Others are the same as those in Embodiment 1 or 2.
specific Embodiment approach 3
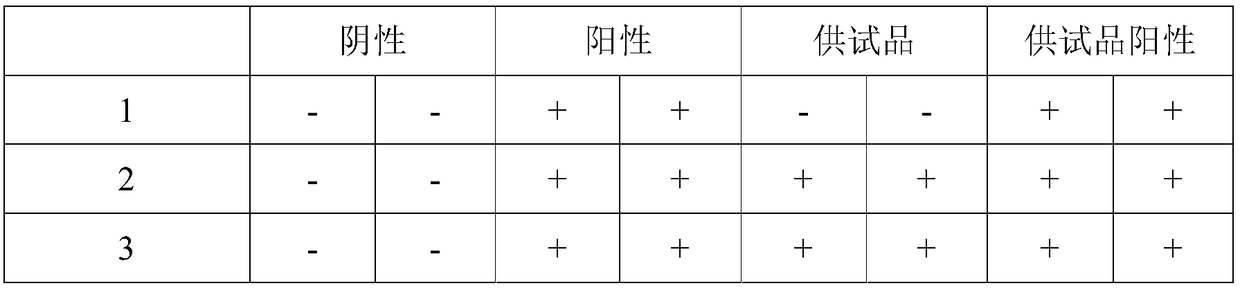
[0014] Specific embodiment three: the difference between this embodiment and specific embodiment one or two is that the diluent B is CaCl with a concentration of 9.0 mg / mL 2 solution. It is the same as the specific embodiment 1 or 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com