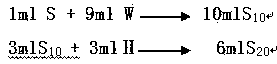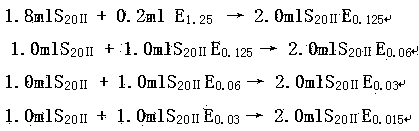Method for detecting bacterial endotoxin in citric acid raw material
A bacterial endotoxin and detection method technology, applied in the field of microbial detection, can solve the problems of inaccurate detection of bacterial endotoxin content, etc., and achieve the effects of high specificity, strong reproducibility, accuracy and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] Step 1, preparation of test solution
[0028] Take 50 mg of citric acid raw material and add it to a test tube, add 10 ml of water for bacterial endotoxin testing, and oscillate until the raw material is fully dissolved, which is the sample solution; dilute the sample solution 10 times with water for bacterial endotoxin testing to generate S 10 solution; then use Na 2 HPO 4 solution will be S 10 The solution is diluted 2 times to generate S 20 solution; diluted S 20 Solution is as need testing solution, i.e. solution A;
[0029] Step 2, preparation of three groups of control solutions
[0030] Prepare the test solution and the bacterial endotoxin standard product into endotoxin solutions containing bacterial endotoxin concentrations of 2.0λ, 1.0λ, 0.5λ, and 0.25λ, respectively, as solution B; each concentration is prepared in parallel with 4 tubes;
[0031] Prepare endotoxin solutions containing bacterial endotoxin concentrations of 2.0λ, 1.0λ, 0.5λ, and 0.25λ wit...
Embodiment 1
[0038] At present, the bacterial endotoxin inspection of raw materials is mainly carried out with reference to the standards of the "Chinese Pharmacopoeia". The raw materials of citric acid are not recorded in the Chinese Pharmacopoeia. The bacterial endotoxin limit value of citric acid raw material in "British Pharmacopoeia": L<0.5EU / mg. Establish internal control standards for citric acid raw materials, set the bacterial endotoxin limits as L<0.25EU / mg and L<0.125EU / mg respectively, and compare the test results.
[0039] Because citric acid is slightly acidic, it is necessary to use pyrogen-free sodium carbonate to weaken its acidity so that the pH value of the test solution is between 6.0-8.0 to meet the requirements of the bacterial endotoxin test method. Considering that pyrogen-free sodium carbonate may affect the test results, NA2HPO4 and pyrogen-free sodium carbonate were selected for the test at the same time, and the test results were compared. Na 2 HPO 4 The sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com