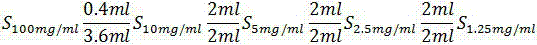Detection method of cysteine hydrochloride bacterial endotoxin
A technology of cysteine hydrochloride and bacterial endotoxin, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of large consumption of LAL reagent, unfavorable LAL protection and sustainable development, sensitivity, detection range, anti-interference ability, Unsatisfactory reliability of test results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] Below in conjunction with embodiment the present invention will be further described.
[0028] 1. Experimental materials
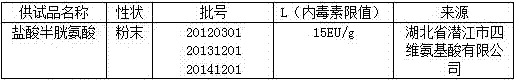
[0029] 1.1 Test product
[0030]
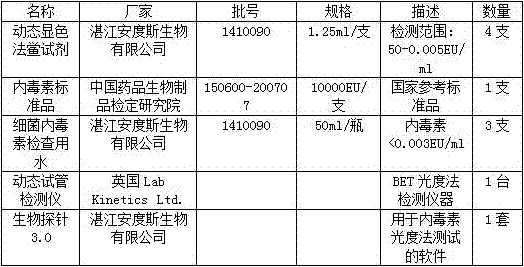
[0031] 1.2 Reagents and instruments
[0032]
[0033] 2. Selection of standard curve and determination of minimum effective concentration
[0034] 2.1 Selection of standard curve (see "Chinese Pharmacopoeia" 2015 edition)
[0035] Assuming that the maximum filling volume of each injection is 20mL, the maximum concentration of cysteine hydrochloride antioxidant used is 0.2%, and the clinical safety factor is 10 times, the amount of antioxidant used in one clinical application is 400mg. Endotoxin limit L = K / M =5 EU / kg÷( 400 mg÷60 kg) =0.75EU / mg.
[0036] In this test, 5mg / ml, 2.5mg / ml, and 1.25mg / ml were selected for the preliminary screening test of interference, so the basis. Endotoxin limit value, the maximum amount of endotoxin that may be contained in the series of reaction solutions is 0.75EU / mg×...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com