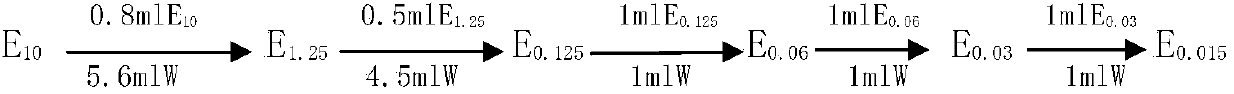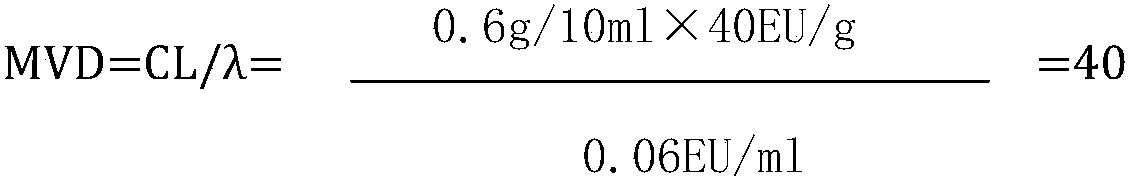Method for testing bacterial endotoxin of ferric citrate pyrophosphate raw material
A technology of pyrophosphate citric acid and bacterial endotoxin, which is applied in the direction of analyzing materials, measuring devices, instruments, etc., can solve problems such as the difficulty in designing bacterial endotoxin inspection methods, and achieve scientific test methods, broad application prospects, and accurate test results Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] The present invention will be further described below in conjunction with embodiments.
[0031] 1. Test preparation
[0032] 1. Test product: raw material of pyrophosphate iron citrate
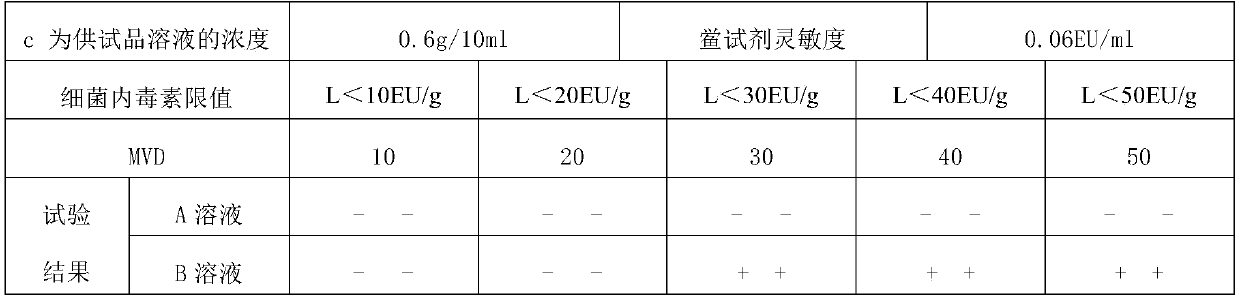
[0033] 2. Limulus reagent: Sensitivity: 0.06EU / ml (purchased from Zhanjiang Andus Biological Co., Ltd.)
[0034] 3. Bacterial endotoxin standard product: potency: 10EU / piece (purchased from Zhanjiang Andus Biological Co., Ltd.)
[0035] 4. Bacterial endotoxin test water (purchased from Zhanjiang Andus Biological Co., Ltd.)
[0036] 5. Test equipment (see Table 1 for details)
[0037] Table 1 Test equipment
[0038] equipment name
factory
Lot number / model
Zhanjiang Andus
TAL-40C
Shanghai Medical University
XW-80A
High temperature oven
YAMATO
DKN612C
Precision pipette
Shanghai refinement
Specification: 50μl~250μl
Electronic balance
METTLER
PR502
[0039] 2. Establishment of test method
[0040] 1. Preparation of solution series
[0041] 1.1 Pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com