Kit for detecting multiple influenza viruses by polymerase chain reaction (PCR) microarray
An influenza virus and microarray technology, applied in the determination/inspection of microorganisms, DNA/RNA fragments, fluorescence/phosphorescence, etc., can solve problems such as antibody-specific interference, and achieve the goal of reducing pollution, workload, and working time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 PCR Microarray Detects Various Influenza Virus Nucleic Acid Kits and Its Use
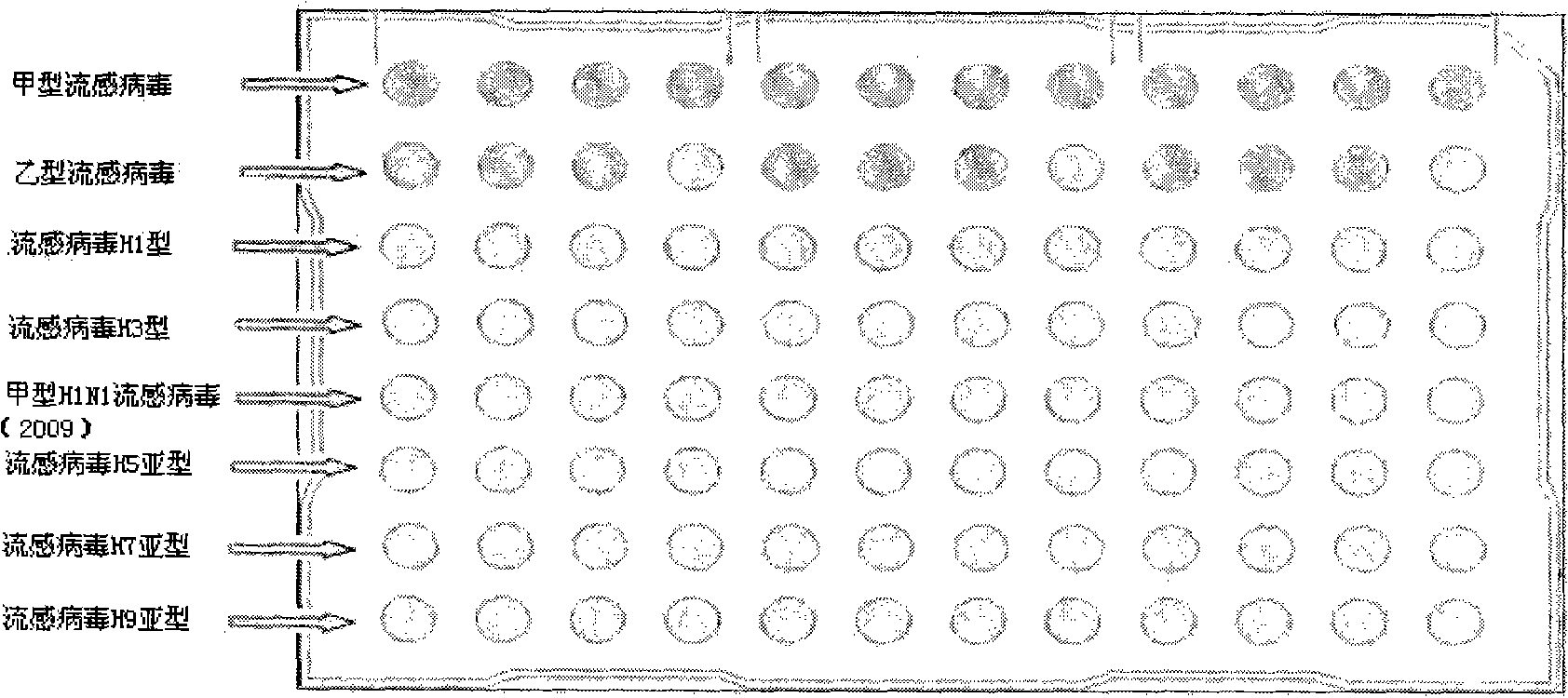
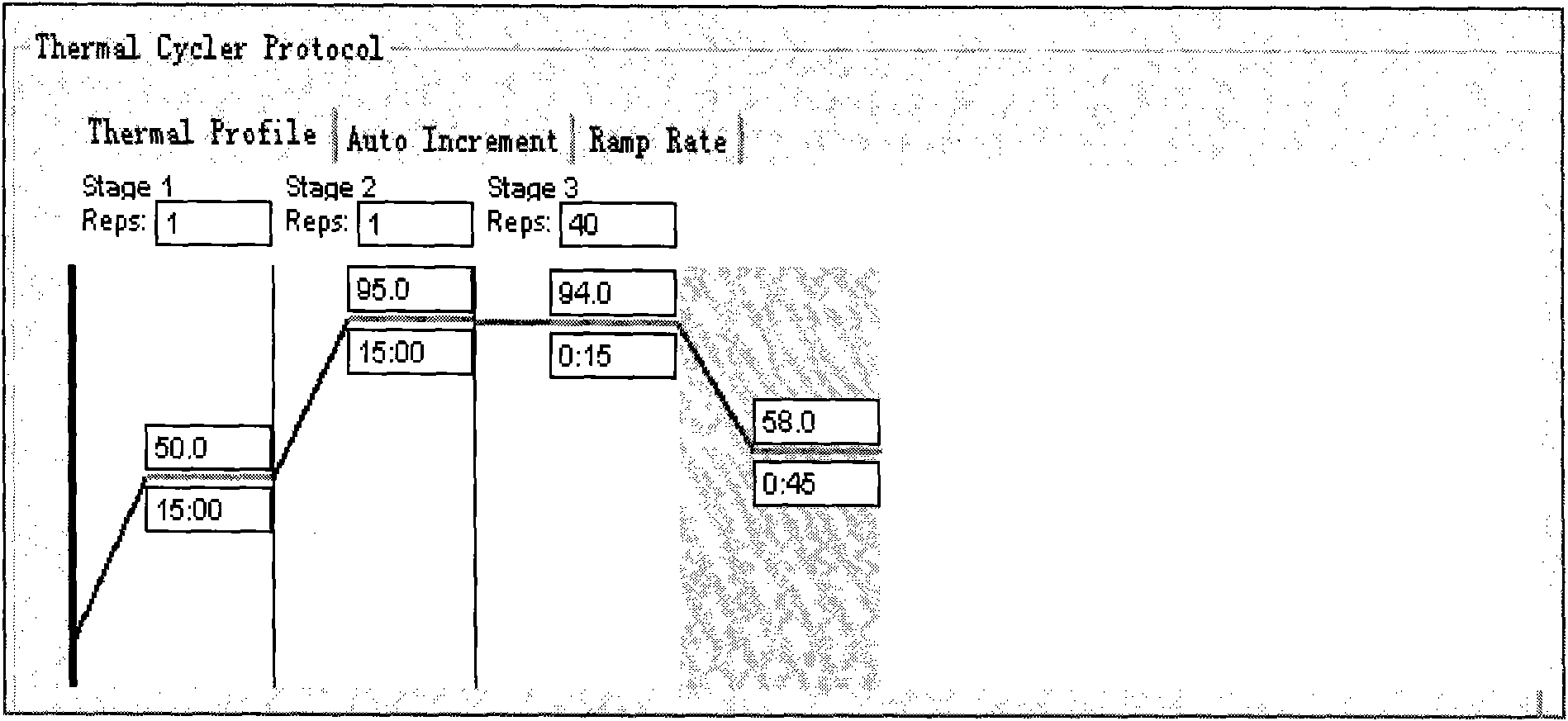
[0038] 1. Prepare a kit including the following components: 1 piece of 96-well PCR microarray reaction plate, 2 tubes of PCR reaction solution, 900 μl / tube.
[0039] 2. Collection, preservation and transportation of specimens:
[0040] 2.1 Applicable specimen types: nasal swab, throat swab, nasopharyngeal aspirate
[0041] 2.2 Specimen collection, storage and delivery:
[0042] It should be collected as soon as possible after the onset of the disease. The specific operation method is as follows: use a sterilized cotton swab to take nasal or throat secretions, and immediately put them into a small test tube containing 2 mL of sterilized normal saline or Eagle solution or 0.5% hydrolyzed milk protein Hanks solution. Label it well, put it in an ice bottle and carry it to the laboratory or freeze it at low temperature (-20℃~-70℃). Dry ice is used for long-distance transport of specim...
Embodiment 2
[0051] Example 2 Application of PCR Microarray to Detect Various Influenza Virus Nucleic Acid Kits to Detect Clinical Samples
[0052] Select 8 cases of throat swab specimens tested as negative for influenza virus by serological methods, and 9 cases as positive for corresponding influenza virus by serological methods, nucleic acid extraction, PCR amplification and result analysis are carried out with reference to Example 1, and negative, Detection of positive quality controls.
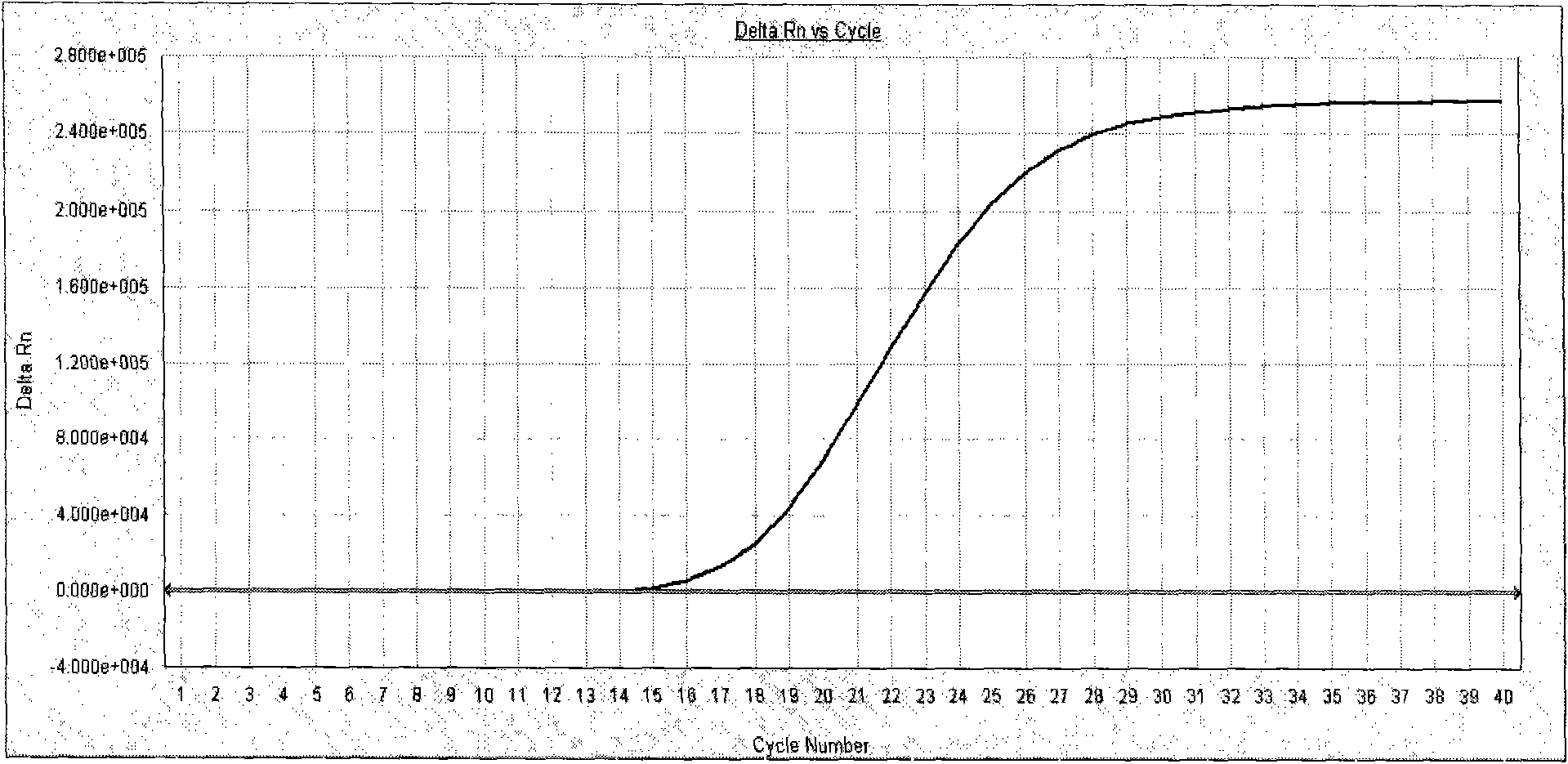
[0053] Interpretation of the test results: the amplification curves of the negative quality control products of each influenza virus are irregular curves or have no CT value; The amplification of the corresponding influenza virus nucleic acid was detected, and the test results of the negative samples were all negative (see attached Figure 3-Figure 12 ).
[0054] The detection results of 17 specimens in this test were completely consistent with the ELISA identification results, indicating that it is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com