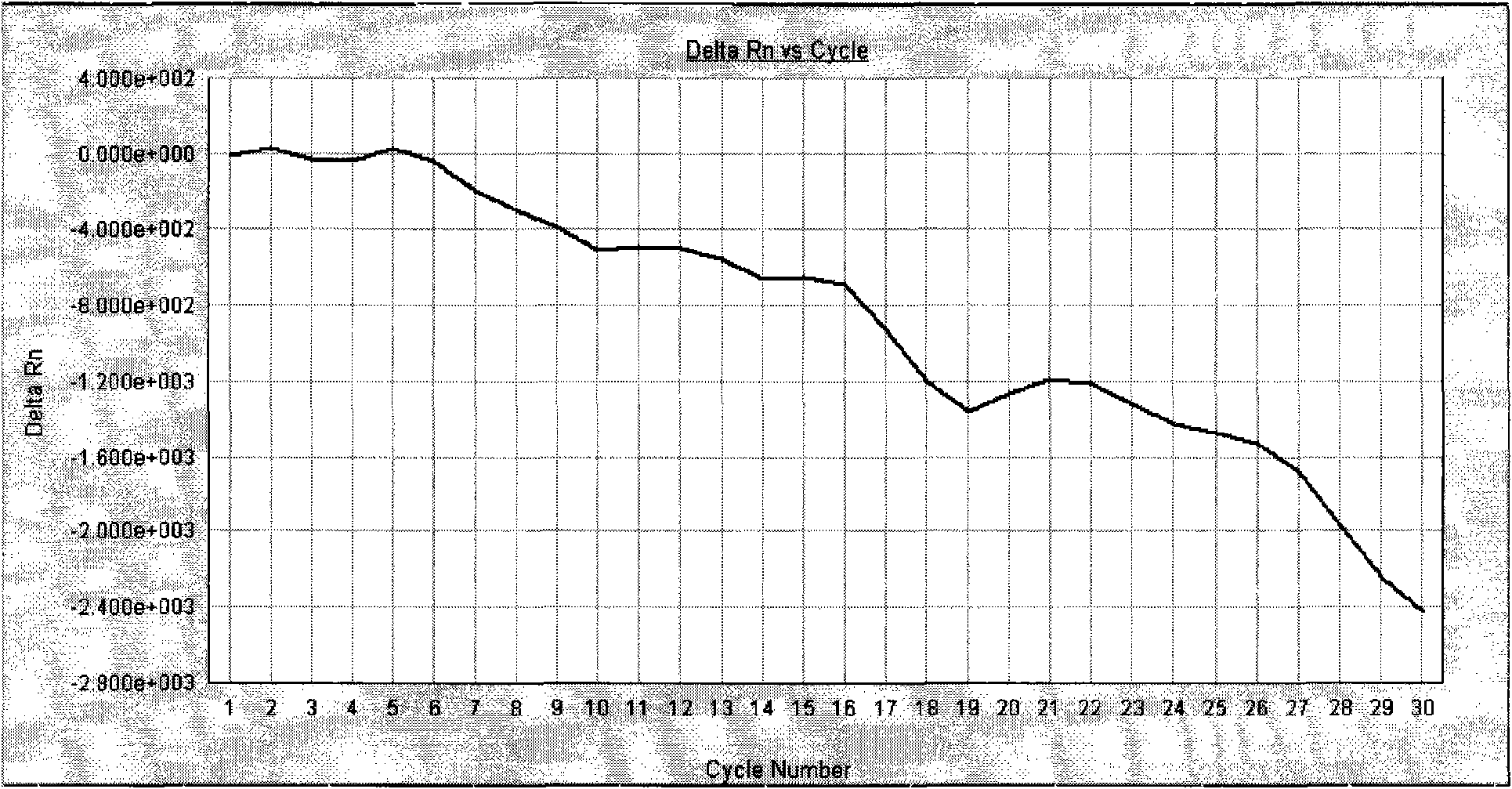
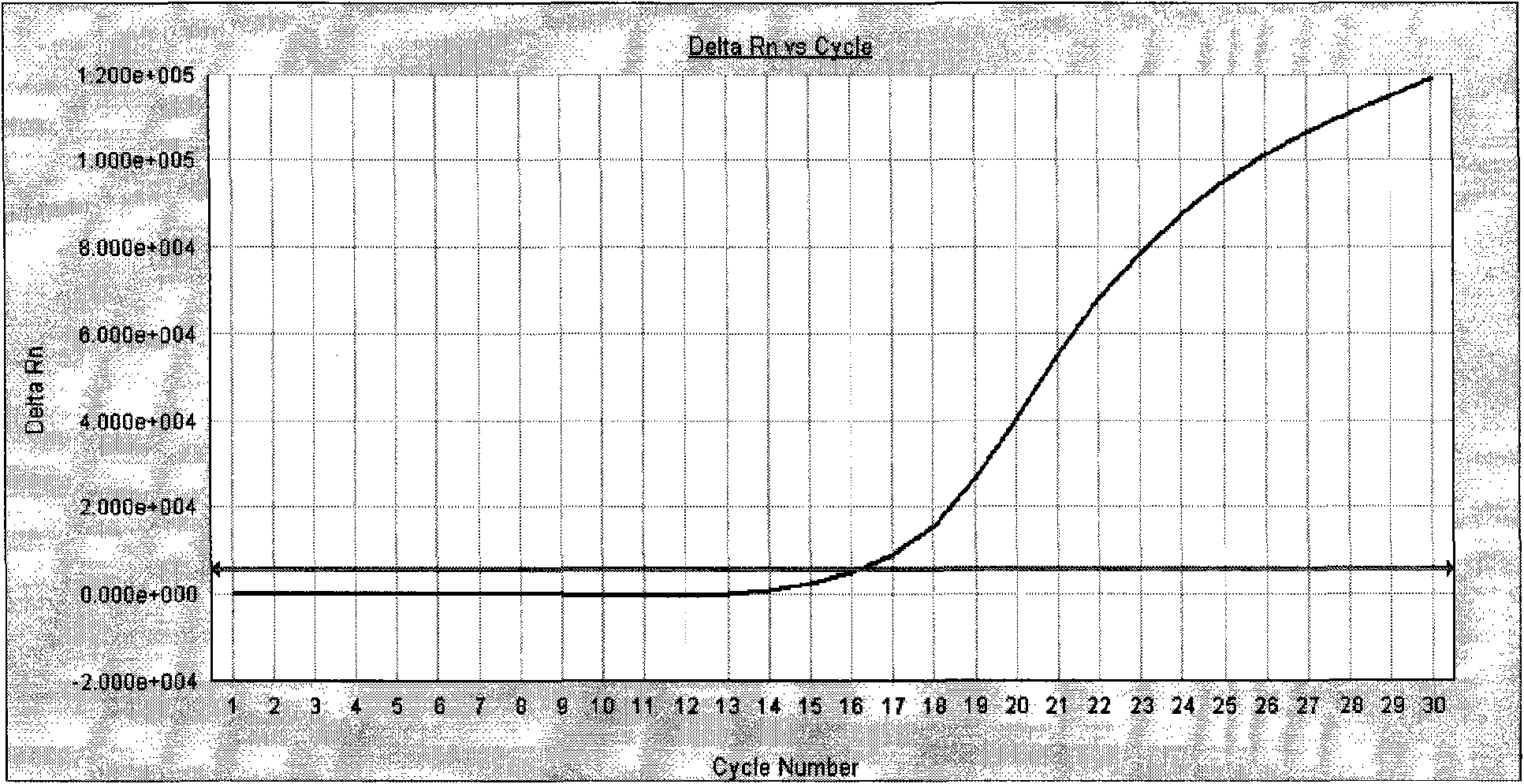
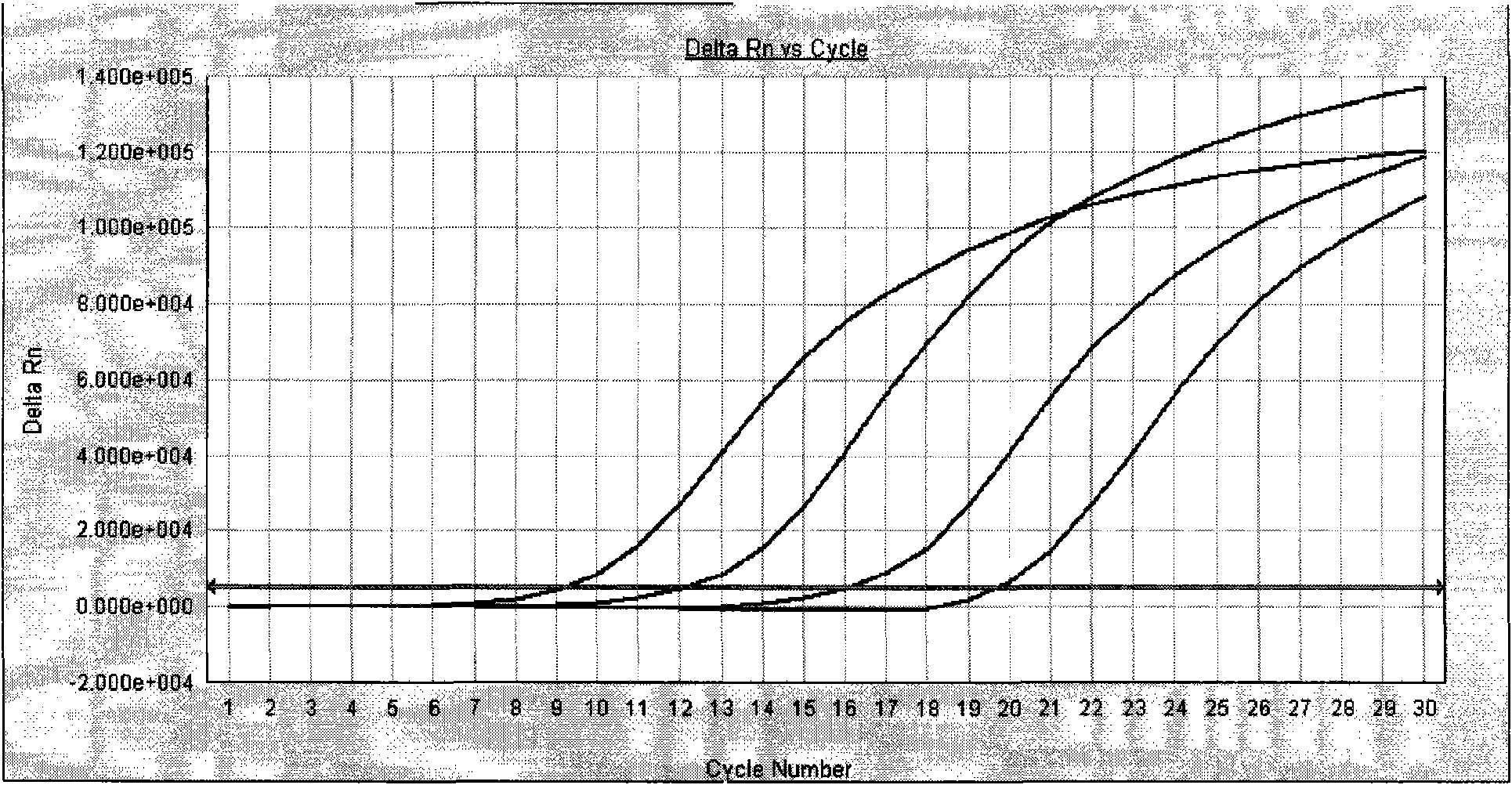
Quantitative detection kit for helicobacter pylori nucleic acid
A technology of Helicobacter pylori and detection kits, which is applied in the field of real-time fluorescent polymerase chain reaction detection kits for Helicobacter pylori, can solve the problems of difficulty in popularization, affect the detection results, false positives, etc., and avoid false positives and environmental pollution. , to avoid cross-reaction, the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1. Composition of Helicobacter pylori Nucleic Acid Quantitative Detection Kit
[0025] 1.1 Extraction reagent: DNA extraction solution: composed of NaOH, Tris-HCl (PH8.0), Chelex-100, etc.
[0026] 1.2PCR Reagent: see PCR reaction tube for composition
[0027] 1.3 Quality control: HP positive quality control; negative quality control; HP quantitative reference (10 5 copies / ml~10 8 copies / ml DNA extracted from HP standard strain)
Embodiment 2
[0028] Example 2. Using the Helicobacter pylori nucleic acid quantitative detection kit to detect the infection of Helicobacter pylori in the sample to be tested
[0029] 2.1 Collection, preservation and transportation of specimens:
[0030] 2.1.1 Applicable specimen types: gastric mucosal tissue biopsy specimens.
[0031] 2.1.2 Specimen collection: Professional physicians take 2 to 3 mucous membranes of suspicious lesions under direct vision of the gastroscope, put them into sterile glass tubes, and send them airtight for inspection.
[0032] 2.1.3 Specimen storage and delivery: Specimens can be used for testing immediately, or stored at -20°C for testing, and the storage period is 12 months. Transport using 0°C ice cubes.
[0033] 2.2 Nucleic acid extraction:
[0034] ①Take the mucosal tissue into a 1.5ml centrifuge tube, cut the tissue into pieces with ophthalmic scissors; thaw the negative and positive quality control products, mix well, centrifuge at 2000rpm for 30sec,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com