Application of karyosome protein SP110 and kit containing karyosome protein SP110 to preparation of early diagnosis reagent for alcoholic cardiomyopathy
An alcoholic cardiomyopathy and nuclear protein technology, applied in the field of medicine, can solve the problem that the diagnosis of alcoholic cardiomyopathy cannot achieve early high sensitivity and high specificity, and cannot achieve early high sensitivity and high specificity of alcoholic cardiomyopathy. Diagnose and other problems to achieve highly specific results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Determination of Candidate Serum Biomarkers
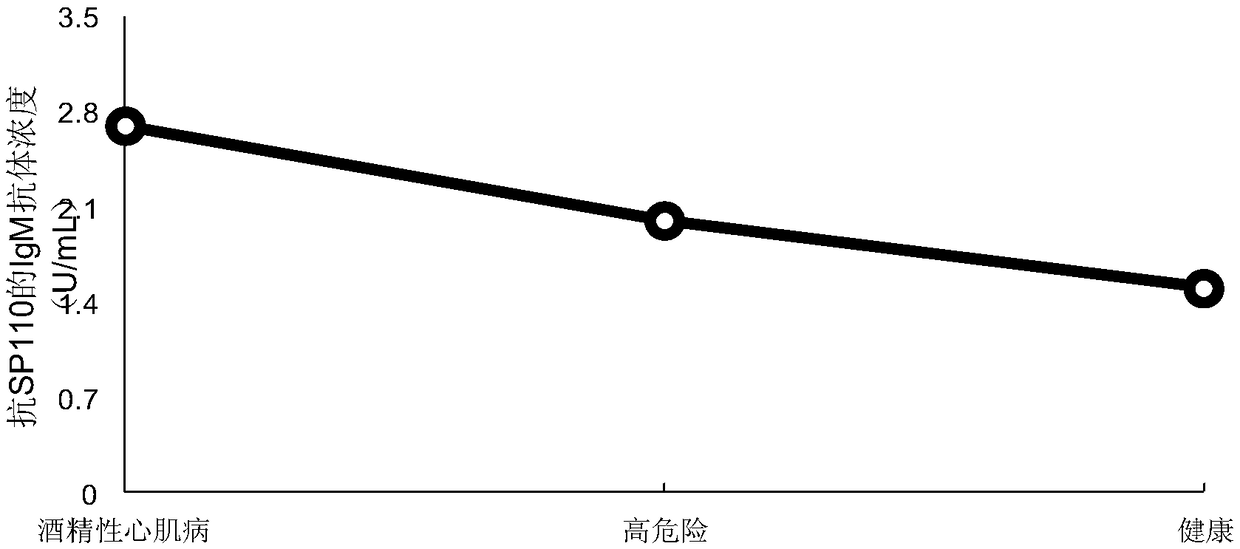
[0044] The present invention utilizes the advantages of high-throughput and rapid analysis of the human proteome chip to analyze 120 serums related to alcoholic cardiomyopathy patients (40 serums from alcoholic cardiomyopathy patients, 20 serums from DCM patients, and 60 serums from healthy people). The differences in the samples of alcoholic cardiomyopathy patients, DCM patients and healthy people were compared, and it was found that the expression level of nuclear body protein Sp110 (Q9HB58-SP110_HUMAN) was significantly higher in alcoholic cardiomyopathy patients than in DCM patients and healthy people. It is suggested that nuclear body protein Sp110 can be used as a candidate serum biomarker of alcoholic cardiomyopathy for early diagnosis and effective treatment of ACM.
Embodiment 2
[0045] Example 2 Application of nucleosomal protein Sp110 in the diagnosis of alcoholic cardiomyopathy
[0046] 1. Expression, purification and identification of nuclear protein Sp110
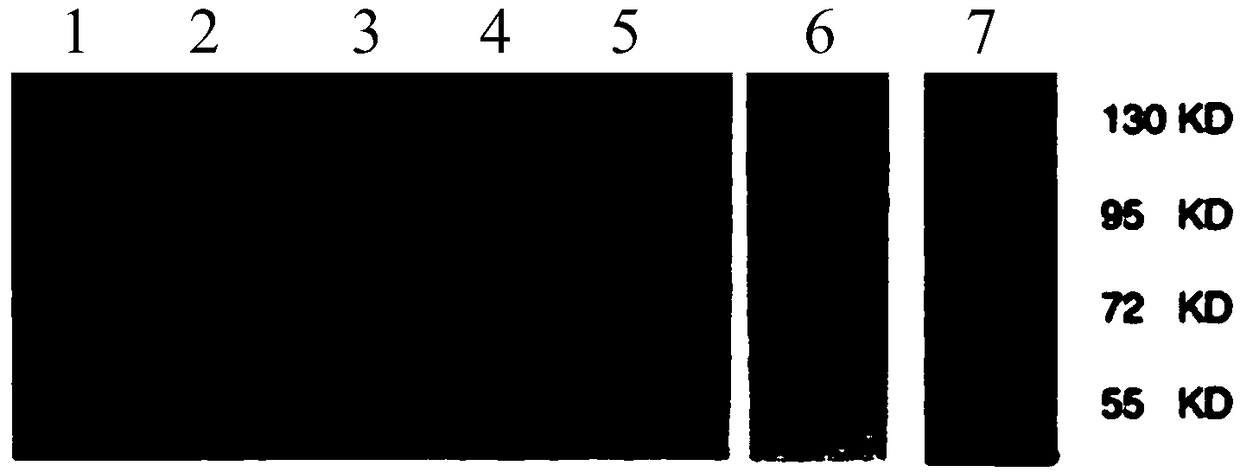
[0047] Human ribosomal protein Sp110 (Q9HB58-SP110_HUMAN, containing 689 amino acids, 78.4KDa) is isolated and purified by agarose affinity medium (glutathione) after overexpression induced by genetically engineered Saccharomyces cerevisiae And obtain, carry out the result of silver staining quantification and Western-Blotting identification to it respectively as follows figure 1 and figure 2 shown.
[0048] 2. Preparation of serum samples:
[0049] Whole blood samples should be left at room temperature for 2 hours, centrifuged at 2000g for about 5 minutes, and the supernatant can be taken for immediate detection; or aliquoted, and the samples should be stored at -20°C or -80°C, but repeated freezing and thawing should be avoided. Thawed samples should be centrifuged again prior to testing...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com