Human immunodeficiency virus neutralizing antibodies adn methods of use thereof
An antibody and virus technology, which is used in the preparation and application of broad-spectrum neutralizing antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0161] Materials, Methods and Instruments
[0162] sample. Human samples were collected from all participating institutions after signing informed consent according to protocols evaluated by the Institutional Review Board (IRB). Patient 1 was selected from a cohort of long-term non-progressors at the Aaron Diamod Aids Research Center in New York. Patients 3 and 8 were selected from an elite group of controllers at the Ragon Institute in Boston. Patients 1, 3 and 8 were selected based on their broad-spectrum neutralizing serum activity against a standard set of HIV isolates. Patient 12 was selected from the Protocol G Cohort of the International Aids Vaccine Initiative based on broad-spectrum serum neutralizing activity.
[0163]Staining, single cell sorting and antibody cloning. Staining and single cell sorting of 2CC nuclear and gp140-specific Ig+ memory B cells were performed (J.F. Scheid et al., Nature 458, 636 (Apr2, 2009)). Briefly, CD19+ B cells were enriched from p...
Embodiment 2
[0179] Isolate HIV Antibody
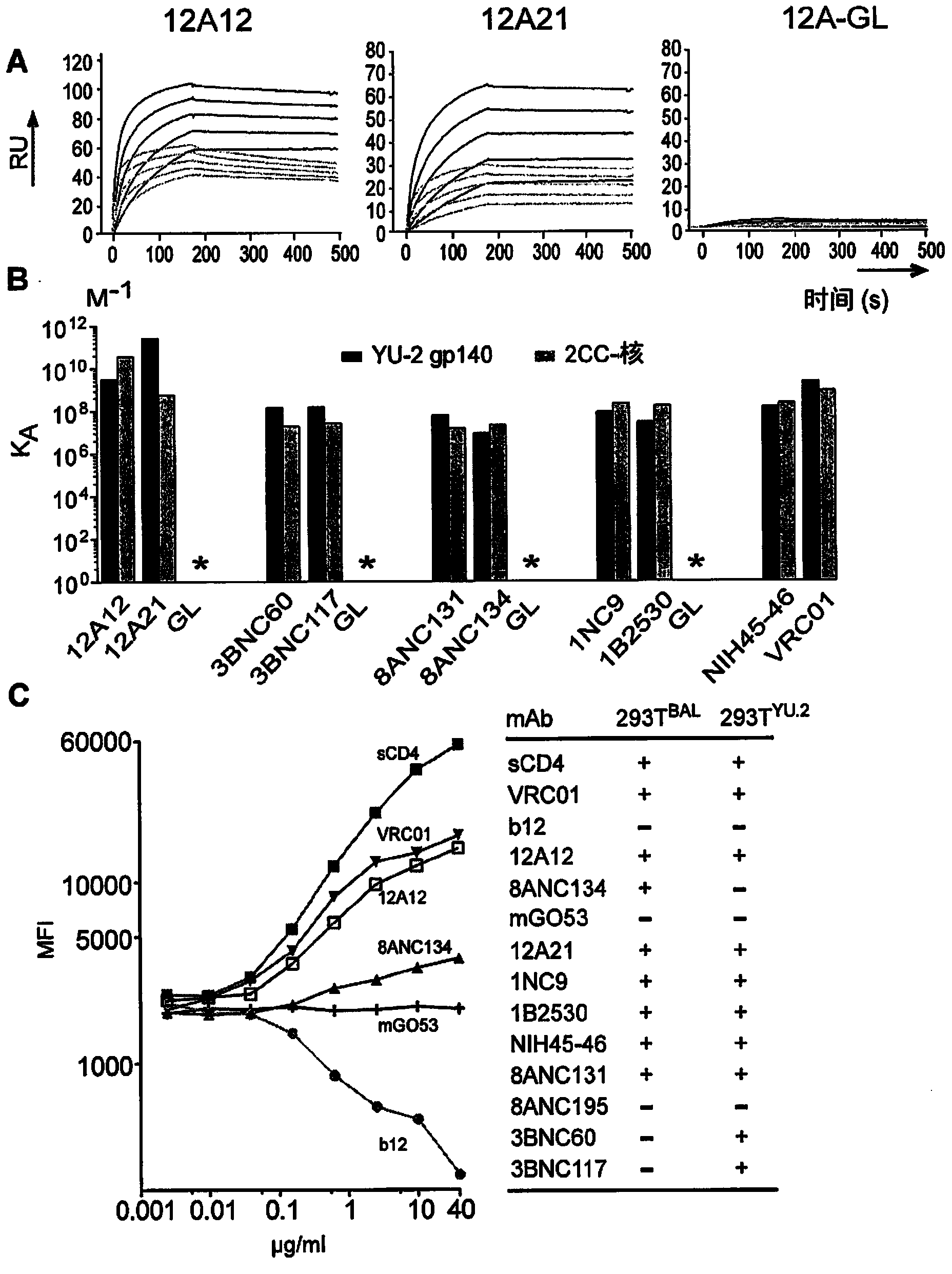
[0180] To determine whether HIV antibody cloning is restricted by somatic mutation, a series of new primers were designed to remove this potential problem (Table 1). The new primer set was tested by sorting B cells bound to the HIV-gp120 nucleoprotein (2CC nucleus) lacking the V1-3 loop and containing a pair of stabilizing disulfide bonds. In contrast to the re-surfaced bait used to clone VRCOl, the 2CC nuclear bait also allowed capture of antibodies against the CD4-induced co-receptor binding site (CD4i).
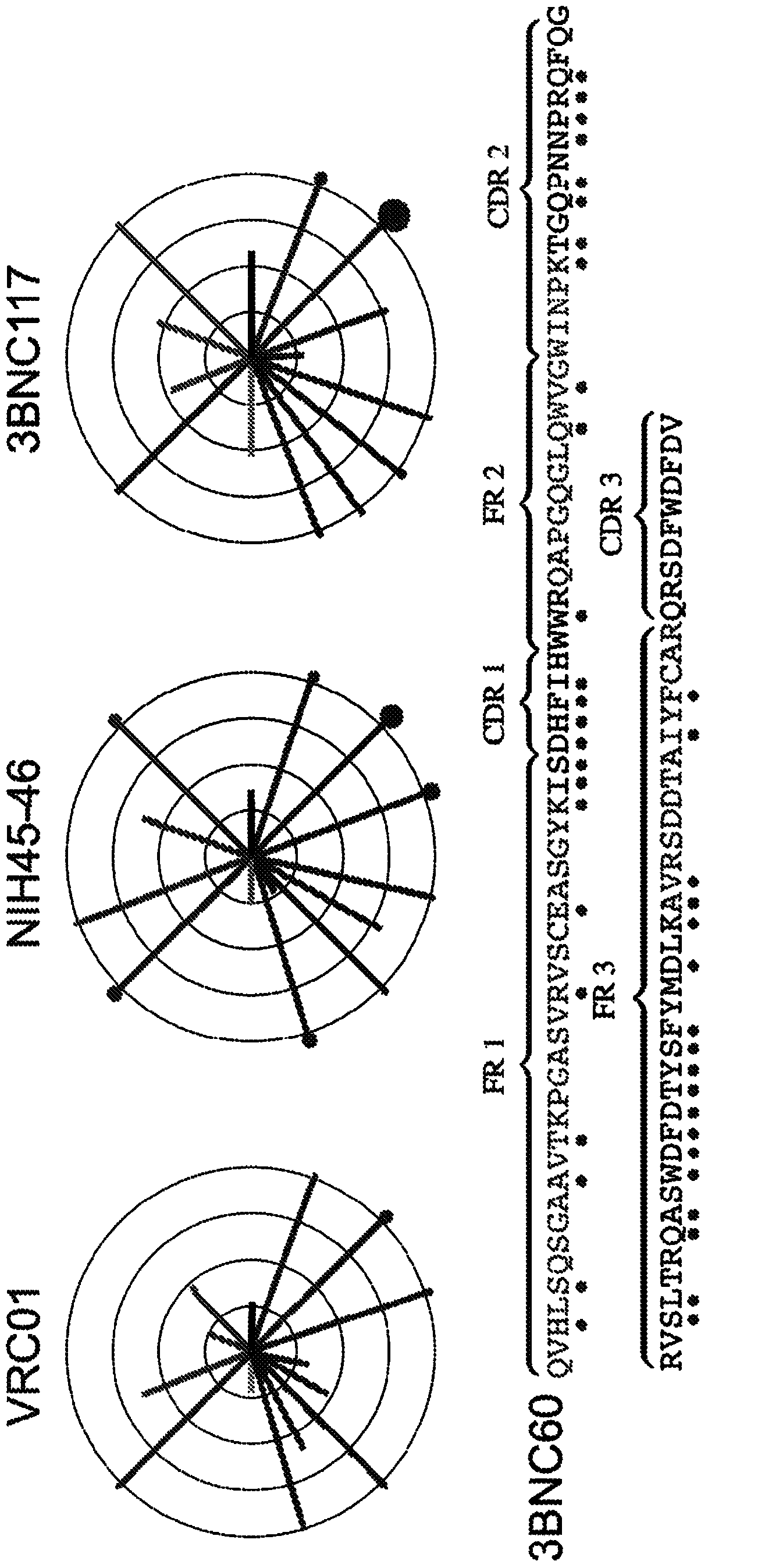
[0181] In comparison, the new primer set increased the recovery of the IgH chain when compared to the original primer set (Fig. 4(a)). Antibodies obtained with the new primer set had more mutations (average 35.6 vs 19.8p=<0.0001 and a maximum of 85 vs 50 for IgH) and contained clones not found with the original primer set (Fig. 4(a)) . To determine whether the new primers could rescue VRCOl-like antibodies from cells that had been sorted wi...
Embodiment 3
[0184] Binding specificity of HIV antibodies
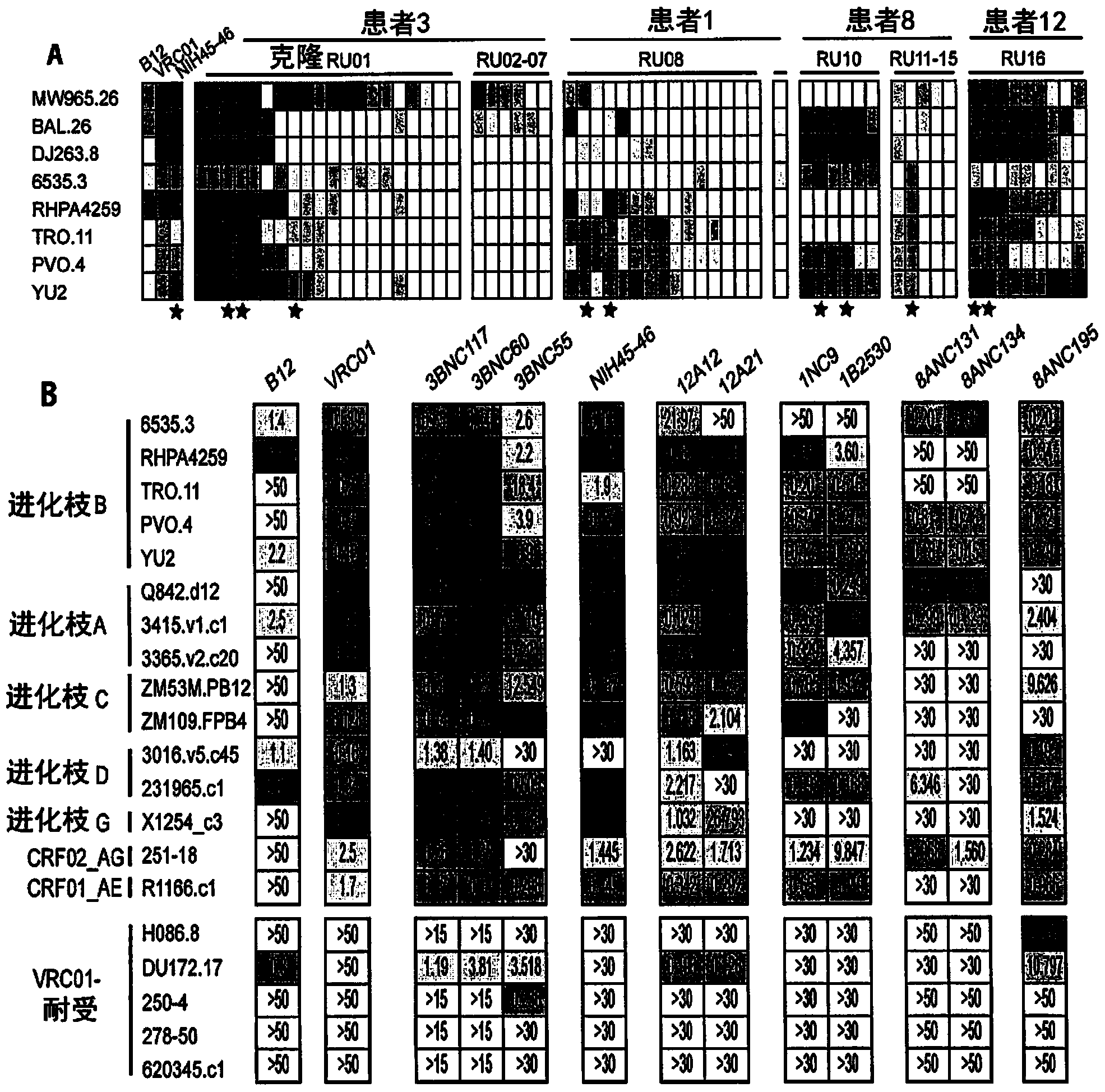
[0185] The antibody clones captured by the 2CC nuclear bait varied widely in size, ranging from 2-76 different members (Table 3). To determine whether antibodies captured by the 2CC core could bind to the HIV spike, an ELISA was performed with YU2gp120 on representative members of each expanded clone. All tested antibodies bound to gp120 (Table 3).
[0186] Sites of antibody binding on the HIV spike were mapped using muteins that interfere with CD4bs (gp120(D368R)) or CD4-induced co-receptor binding sites (CD4i, gp120(I420R)). As reported by X. Wu et al., Science 329, 856 (13 August 2010), since VRC01 is sensitive to the D368R mutation, it is listed as a CD4bs antibody, but due to its proximity to the CD4i site, it also shows some sensitivity to the I420R mutation sensitive. NIH45-46, antibodies 3BNC60, 8ANC131 and 12A12, belonging to VRC01 variants, had ELISA profiles similar to VRC01 (these clone members were selected based o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com