HIV-1 broadly neutralizing antibody and use thereof
An HIV-1, antibody technology, applied in the direction of antibodies, antibody medical components, applications, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
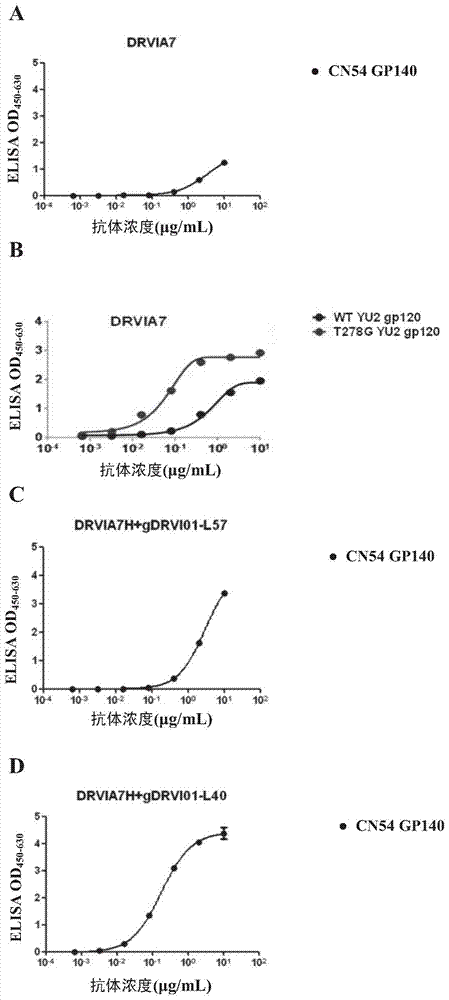
[0077] Example 1: Identification of broad-spectrum neutralizing antibody DRVIA7
[0078] Antibody DRVIA7 isolated from Chinese HIV-1 infected patients
[0079] The inventor isolated a monoclonal antibody from a sample of a Chinese HIV-1 infected person. It has been identified that the antibody has broad-spectrum neutrality to a variety of HIV-1, and it is named DRVIA7. The Escherichia coli carrying the expression vector (DRVIA7H) of the heavy chain gene and the expression vector (DRVIA7L) of the light chain gene containing the antibody DRVIA7 were deposited in the China General Microorganism Culture Collection Center ( China General Microbiological Culture Collection Center, CGMCC) (No. 3, Yard No. 1, Beichen West Road, Chaoyang District, Beijing). The coding sequence of the heavy chain variable region contained in DRVIA7H is SEQ ID NO:1, and the amino acid sequence of the heavy chain variable region encoded by it is SEQ ID NO:2. The coding sequence of the light chain varia...
Embodiment 2
[0098] Example 2: Modified antibody DRVIA7H+gDRVI01-L57
[0099] Amino Acid Residue Based Energy Estimation
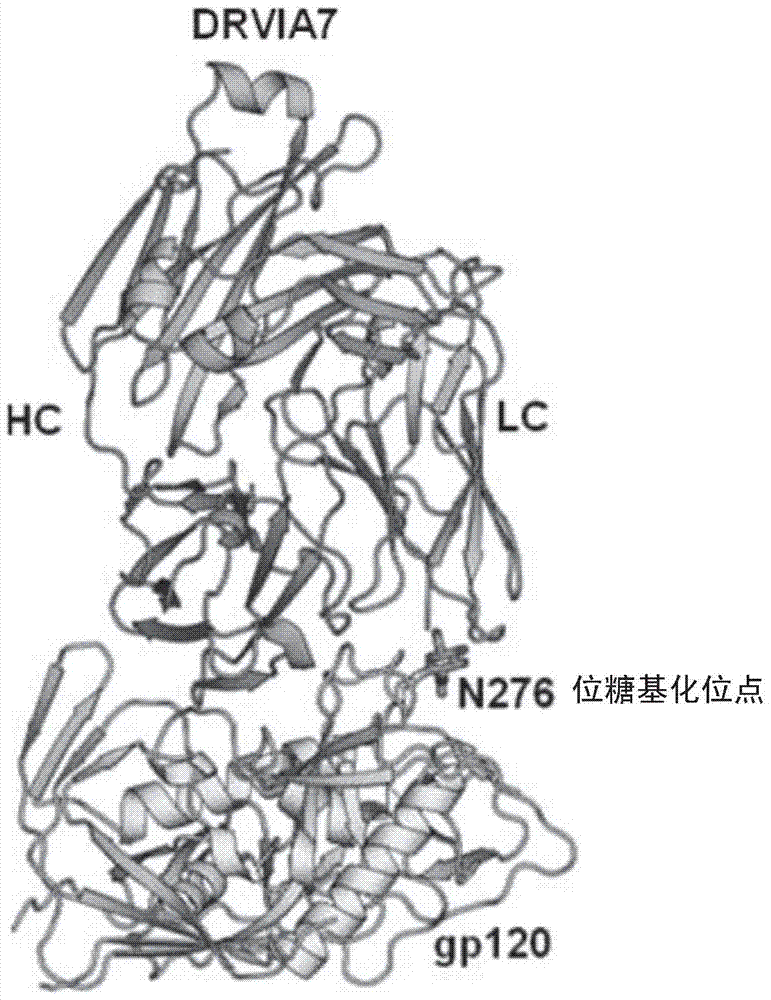
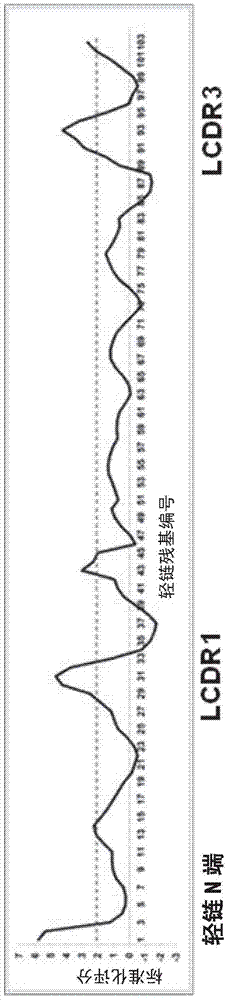
[0100] The inventors performed a residue-based energy evaluation of the complex formed by DRVIA7+gp120 [8] . Such as image 3 As shown, it was found that there may be structural conflicts that interfere with the binding of DRVIA7 to gp120 at the N-terminal, CDRL1 and CDRL3 regions of the light chain variable region of DRVIA7.
[0101] Transformed light chain variable region gDRVI01-L57
[0102] Based on this finding, the inventors made a series of modifications to the light chain of DRVIA7. Various modifications have been made to the CDRL1 region, but the neutralizing ability of the antibody has not been significantly improved. However, when the two N-terminal amino acids of the light chain variable region of DRVIA7 were deleted, it was found that the binding ability and neutralization width of the engineered antibody were greatly improved, and the transformed antib...
Embodiment 3
[0108] Example 3: Antibody DRVIA7H+gDRVI01-L40 was obtained by screening the antibody light chain gene library
[0109] DRVIA7 Antibody Sequence Analysis
[0110] Such as Figure 5 As indicated, the DRVIA7 antibody gene was analyzed using the antibody gene analysis database IMGT V-QEST server (http: / / www.imgt.org / IMGT_vquest / vquest?livret=0&Option=humanIg). The DRVIA7 antibody heavy chain belongs to the IgHV1-02*02 family, and the CDRH3 is 11 amino acids (Kabat nomenclature). The light chain belongs to the IgKV1-5*03 family, and CDRL3 is 5 amino acids. with VRC01 antibody [1] The comparison found that the DRVIA7 antibody and the VRC01 antibody use the same heavy chain family gene, and the length of the light chain CDRL3 is the same as that of VRC01, revealing that the DRVIA7 antibody may belong to the VRC01-like antibody.
[0111] Structural comparative analysis of DRVIA7 and VRC01-like antibodies
[0112] In Example 1, the crystal structure of the DRVIA7 antibody was res...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com