Patents
Literature
73 results about "Interleukin 18" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Interleukin-18 (IL18, also known as interferon-gamma inducing factor) is a protein which in humans is encoded by the IL18 gene. The protein encoded by this gene is a proinflammatory cytokine. Many cell types, both hematopoietic cells and non-hematopoietic cells, have the potential to produce IL-18. It was first described in 1989 as a factor that induced interferon-γ (IFN-γ) production in mouse spleen cells. Originally, IL-18 production was recognized in Kupffer cells, liver-resident macrophages. However, IL-18 is constitutively expressed in non-hematopoietic cells, such as intestinal epithelial cells, keratinocytes, and endothelial cells. IL-18 can modulate both innate and adaptive immunity and its dysregulation can cause autoimmune or inflammatory diseases.
Conjugates comprising human IL-18 and substitution mutants thereof
Human interleukin-18 (IL-18) polypeptides and substitution mutants thereof were conjugated to water-soluble polymers at specific sites on the human IL-18 protein. These conjugated human IL-18 and substitution mutants thereof retain biological activity. These conjugated cytokines demonstrate enhanced and unexpected biological properties when compared to the corresponding unconjugated cytokines.
Owner:GLAXO SMITHKLINE LLC
Antibodies that bind IL-18 and methods of inhibiting IL-18 activity
InactiveUS7767207B2Inhibit hIL-1 activityInhibitory activityAntibacterial agentsOrganic active ingredientsEpitopeAntigen binding
Antibodies that bind human interleukin-18 (hIL-18) are provided, in particular antibodies that bind epitope(s) of human IL-18. The antibodies can be, for example, entirely human antibodies, recombinant antibodies, or monoclonal antibodies. Preferred antibodies have high affinity for hIL-18 and neutralize hIL-18 activity in vitro and in vivo. An antibody of the invention can be a full-length antibody or an antigen-binding portion thereof. Method of making and method of using the antibodies of the invention are also provided. The antibodies, or antibody portions, of the invention are useful for detecting hIL-18 and for inhibiting hIL-18 activity, e.g., in a human subject suffering from a disorder in which hIL-18 activity is detrimental.
Owner:ABBVIE INC
Diagnostic Methods Of Multiple Organ Amyloidosis
InactiveUS20080038192A1In-vivo radioactive preparationsMicrobiological testing/measurementElevated C-reactive proteinPredictive biomarker
Described are methods of assessing whether a subject has or is at risk of having multiple organ amyloidosis (MOA) The method includes detecting a diagnostically predictive collection of biomarkers of multiple organ amyloidosis, wherein the detection of a diagnostically predictive collection of biomarkers indicates the subject has or is at risk of having multiple organ amyloidosis Also described are methods of monitoring treatment of subjects with multiple organ amyloidosis and evaluating therapeutic compounds Representative biomarkers for use in the methods may be selected from variant serum amyloid A (SAA) allele, elevated SAA level, elevated C-reactive protein (CRP) level, depressed glycosammoglycan (GAG) level, elevated interleukin-18 (IL-18) level, elevated macrophage-colony stimulating factor (M-CSF) level, elevated hepatocyte growth factor (HGF) level, presence of an antibody against citrullmated vimentm (Sa), presence of a monoclonal immunoglobulin light chain, increased serum albumin, and increased creatinine clearance
Owner:NEUROCHEM INT
Vaccine Compositions Comprising An Interleukin 18 And Saponin Adjuvant System
InactiveUS20070212329A1Enhance immune responseReduce severityBiocidePeptide/protein ingredientsAdjuvantAutoimmune condition
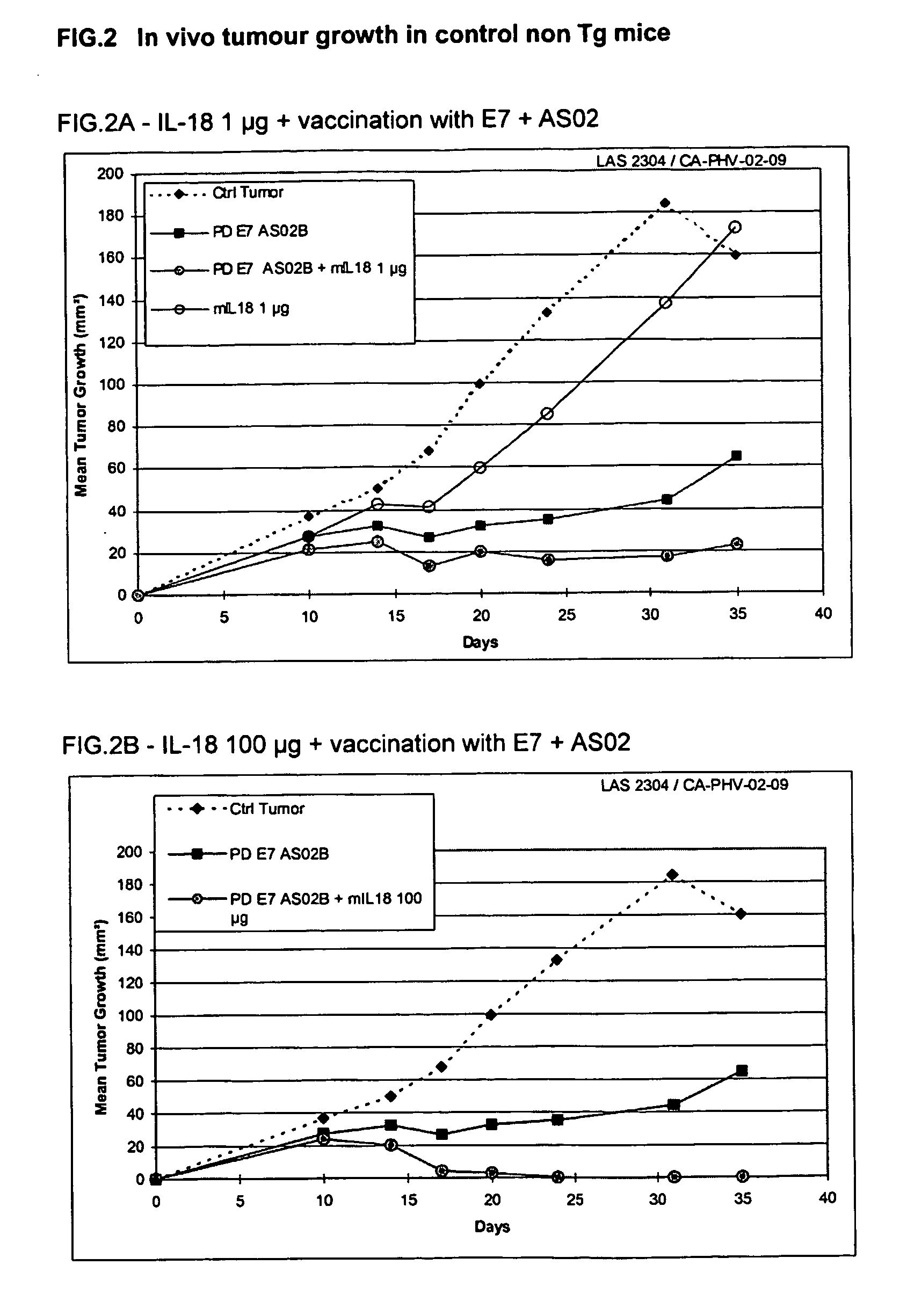
This invention relates to a combination therapy that finds utility in the treatment or prophylaxis of infectious diseases, cancers, autoimmune diseases and related conditions. In particular, the combination therapy comprises the administration of a TH-1 cytokine, in particular, IL-18, and an immunogenic composition, in particular, a vaccine, comprising an antigen and a saponin adjuvant. In particular, the invention relates to the use of IL-18 or bioactive fragment or variant thereof and an immunogenic composition comprising a tumour-associated antigen and a saponin adjuvant, for the treatment of preneoplasic lesions or cancer.
Owner:SMITHKLINE BECKMAN CORP +1
Antibodies that bind il-18 and methods of inhibiting il-18 activity
InactiveUS20100104563A1Inhibitory activityInhibit hIL-1 activityAntibacterial agentsOrganic active ingredientsEpitopeAntigen binding
Antibodies that bind human interleukin-18 (hIL-18) are provided, in particular antibodies that bind epitope(s) of human IL-18. The antibodies can be, for example, entirely human antibodies, recombinant antibodies, or monoclonal antibodies. Preferred antibodies have high affinity for hIL-18 and neutralize hIL-18 activity in vitro and in vivo. An antibody of the invention can be a full-length antibody or an antigen-binding portion thereof. Method of making and method of using the antibodies of the invention are also provided. The antibodies, or antibody portions, of the invention are useful for detecting hIL-18 and for inhibiting hIL-18 activity, e.g., in a human subject suffering from a disorder in which hIL-18 activity is detrimental.
Owner:ABBVIE INC
Conjugates comprising human IL-18 and substitution mutants thereof
ActiveUS20050008615A1Improve pharmacokineticsImproving subcutaneous bioavailabilityPeptide/protein ingredientsPeptide preparation methodsBiological propertyWater soluble
Owner:GLAXO SMITHKLINE LLC
Method for preparing autologous natural killer cell in cocktail culture and and kit product
InactiveCN104789527AMammal material medical ingredientsBlood/immune system cellsPeripheral blood mononuclear cellRecombinant Human Interleukin-15
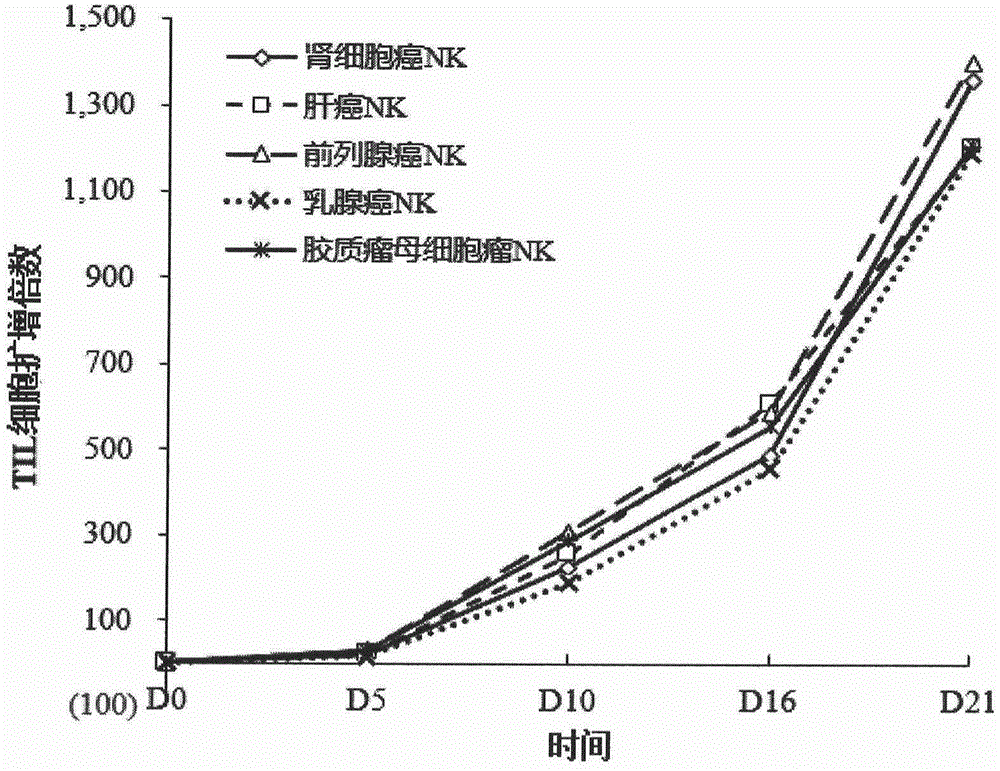
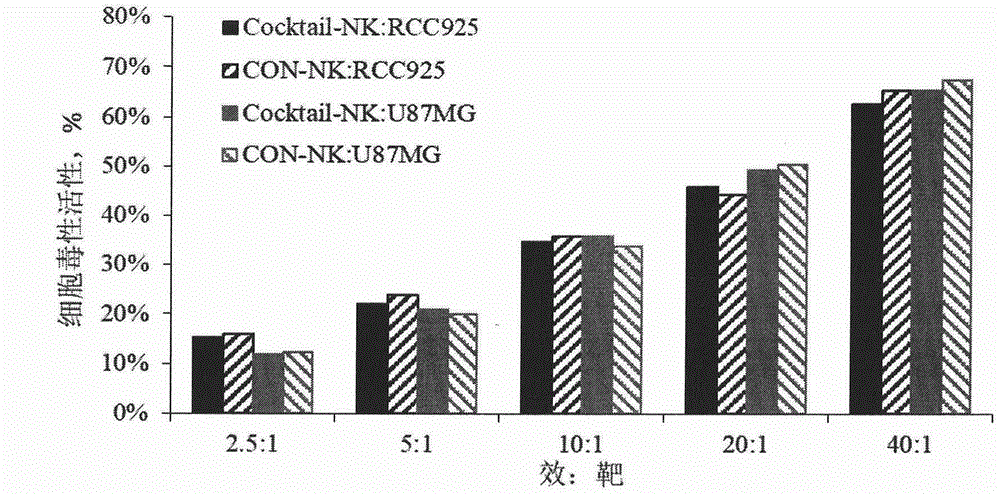
The invention discloses a method for preparing autologous natural killer cell in cocktail culture and a kit product. The method is characterized in that proliferation of natural killer cells of peripheral blood mononuclear cells from a cancer patient is activated under the combined action of a recombinant human interleukin 15, a recombinant human interleukin 18, a recombinant human interleukin 21, a recombinant human interleukin 12, a recombinant human interleukin 7 and a recombinant human MHC-1 chain related molecule A, and the natural killer cell killing potential is reinforced. The invention also discloses a kit containing the autologous natural killer cell in cocktail culture prepared by the method. The kit can be used for clinically acquiring plenty of natural killer cells and performing antitumor and antivirus treatment.
Owner:JIANGSU JIESHENG BIOSCI CO LTD
Anti-il-18 antibodies and their uses
ActiveUS20140004128A1Reduced activityInhibit bindingBacteriaAntipyreticAnti-CEA AntibodyChimeric antibody
The present invention provides human, humanized and / or chimeric antibodies as well as fragments, derivatives / conjugates and compositions thereof with a specific binding affinity for interleukin-18. The invention includes the use of these antibodies for diagnosing and treating diseases associated with increased IL-18 activity, the latter in the form of a pharmaceutical composition.
Owner:MEDIMMUNE LTD
IL-18 binding proteins
The present invention encompasses IL-18 binding proteins, particularly antibodies that bind human interleukin-18 (hIL-18). Specifically, the invention relates to antibodies that are entirely human antibodies. Preferred antibodies have high affinity for hIL-18 and / or that neutralize hIL-18 activity in vitro and in vivo. An antibody of the invention can be a full-length antibody or an antigen-binding portion thereof. Method of making and method of using the antibodies of the invention are also provided. The antibodies, or antibody portions, of the invention are useful for detecting hIL-18 and for inhibiting hIL-18 activity, e.g., in a human subject suffering from a disorder in which hIL-18 activity is detrimental.
Owner:ABBVIE INC
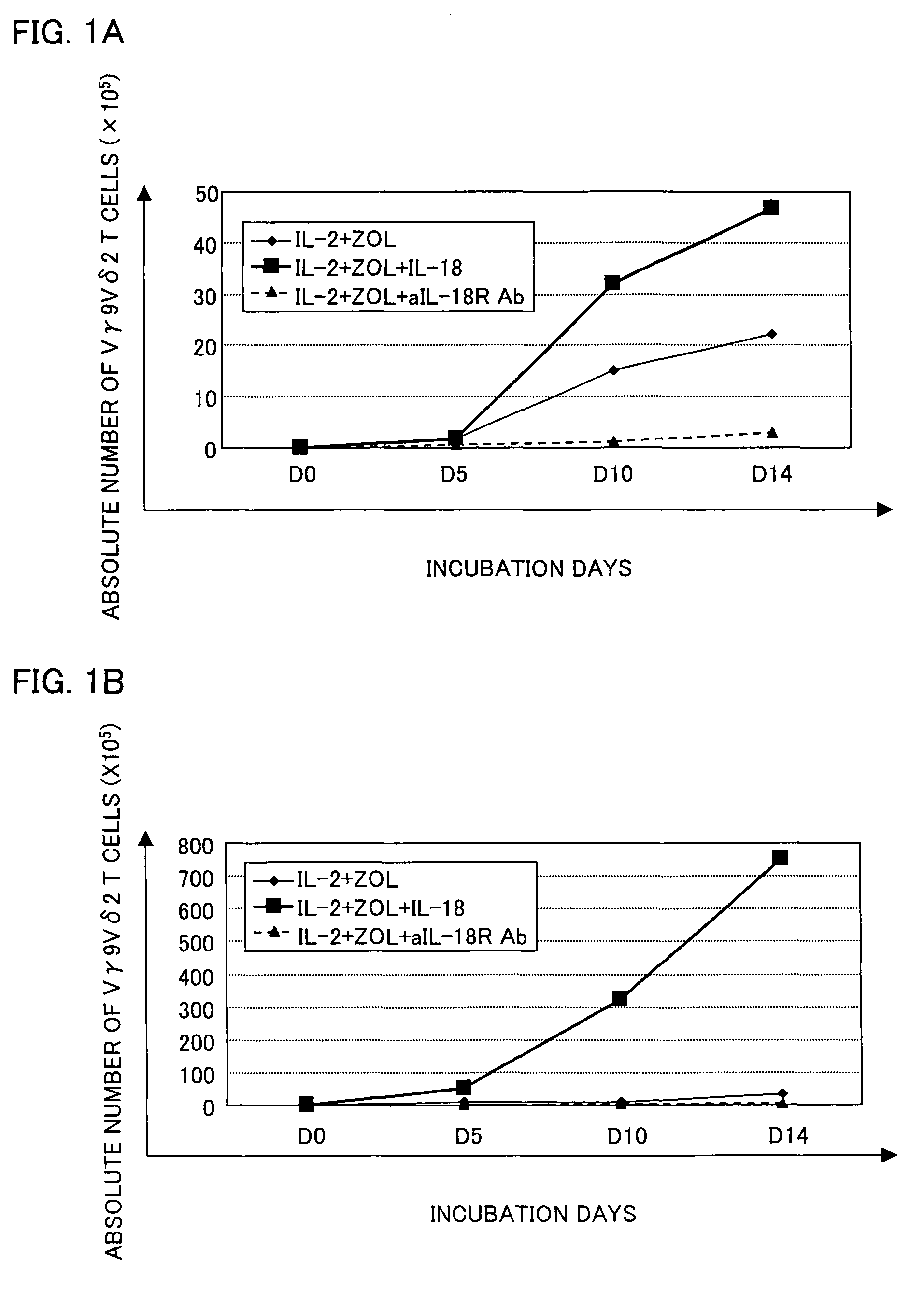
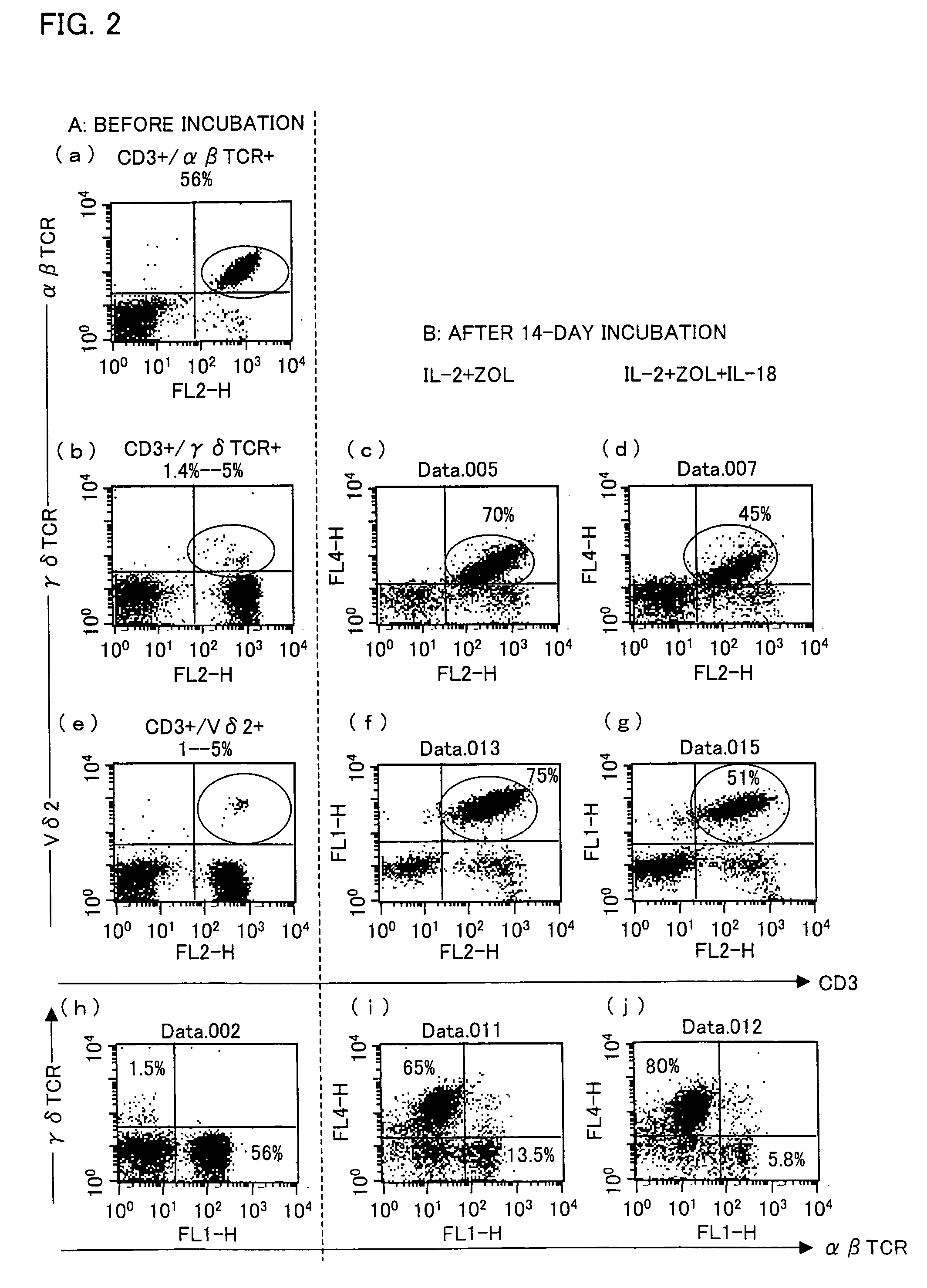
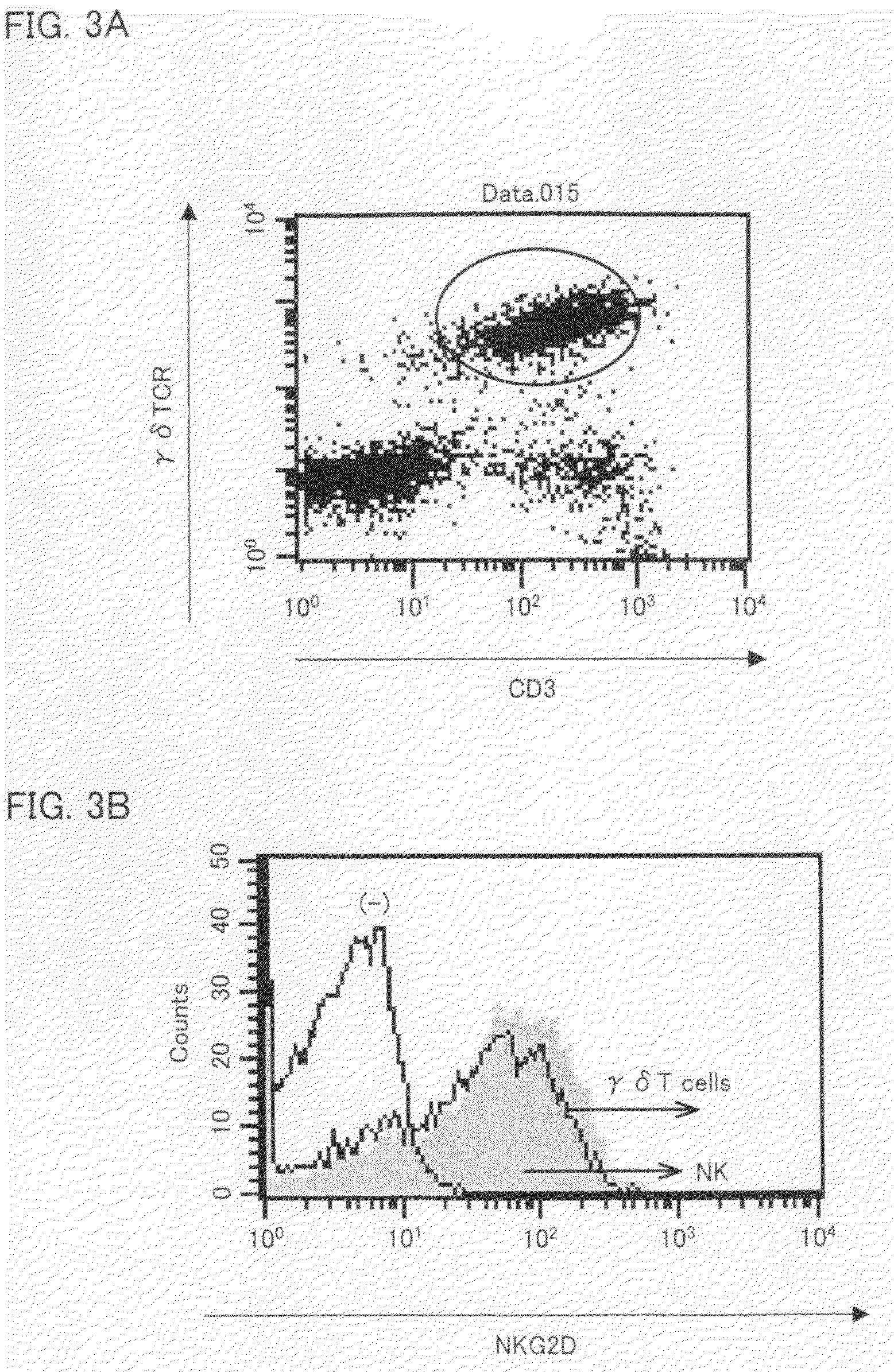
Vγ9Vδ2 T cell proliferation agent, method for producing activated Vγ9Vδ2 T cells, and uses thereof
InactiveUS7749760B2Increase in sizeGood effectOrganic active ingredientsPeptide/protein ingredientsWhite blood cellFactor ii
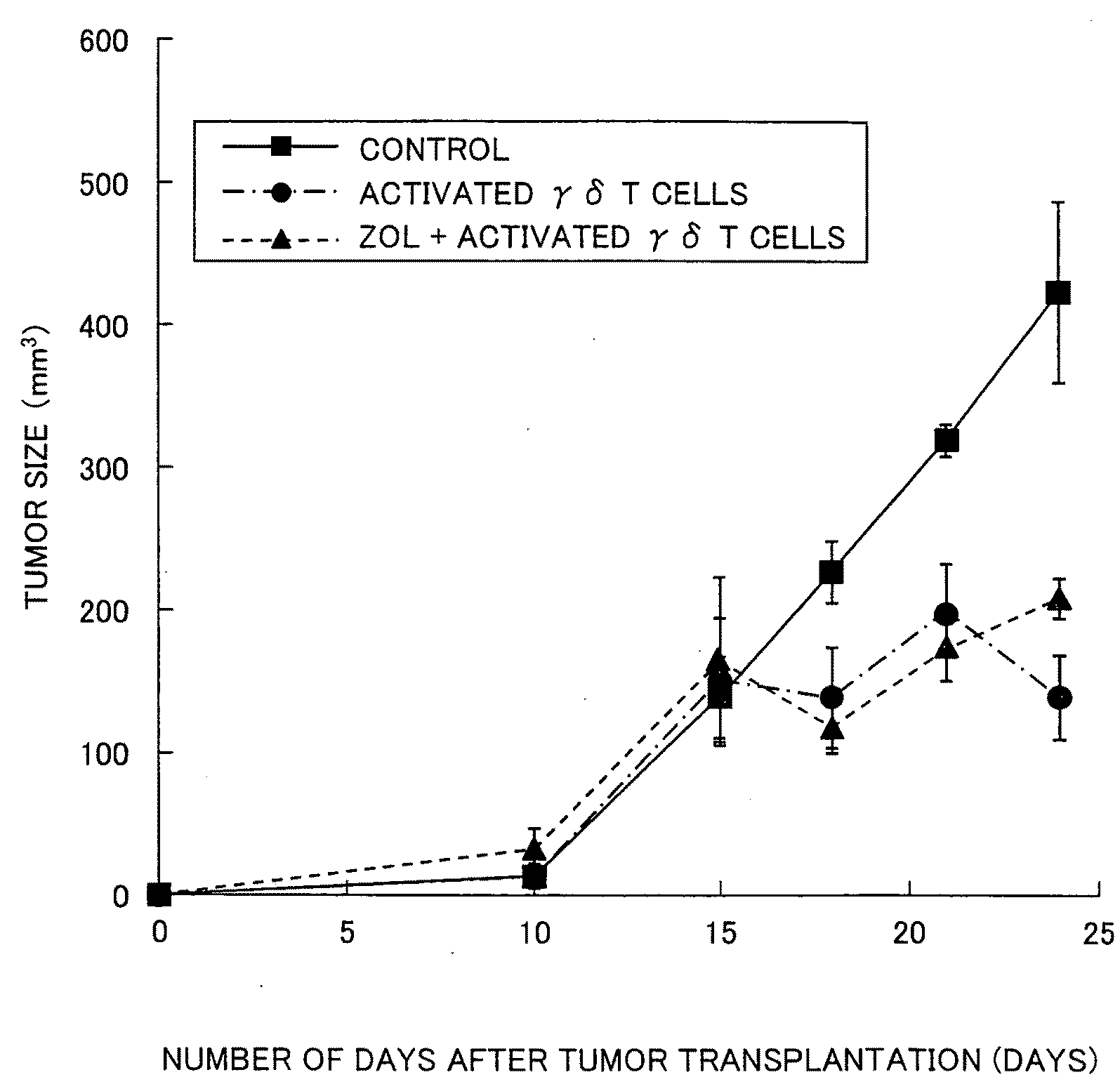
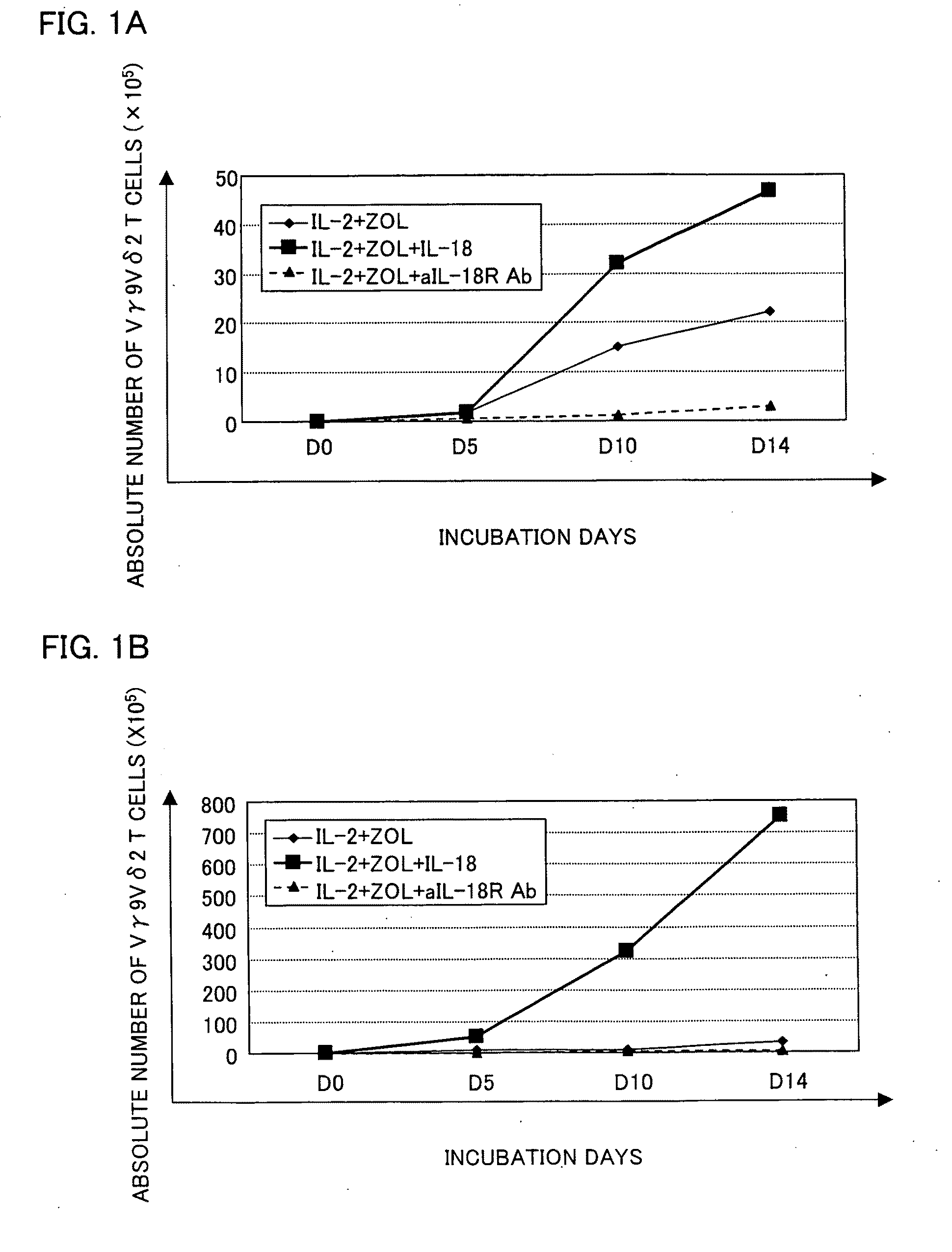
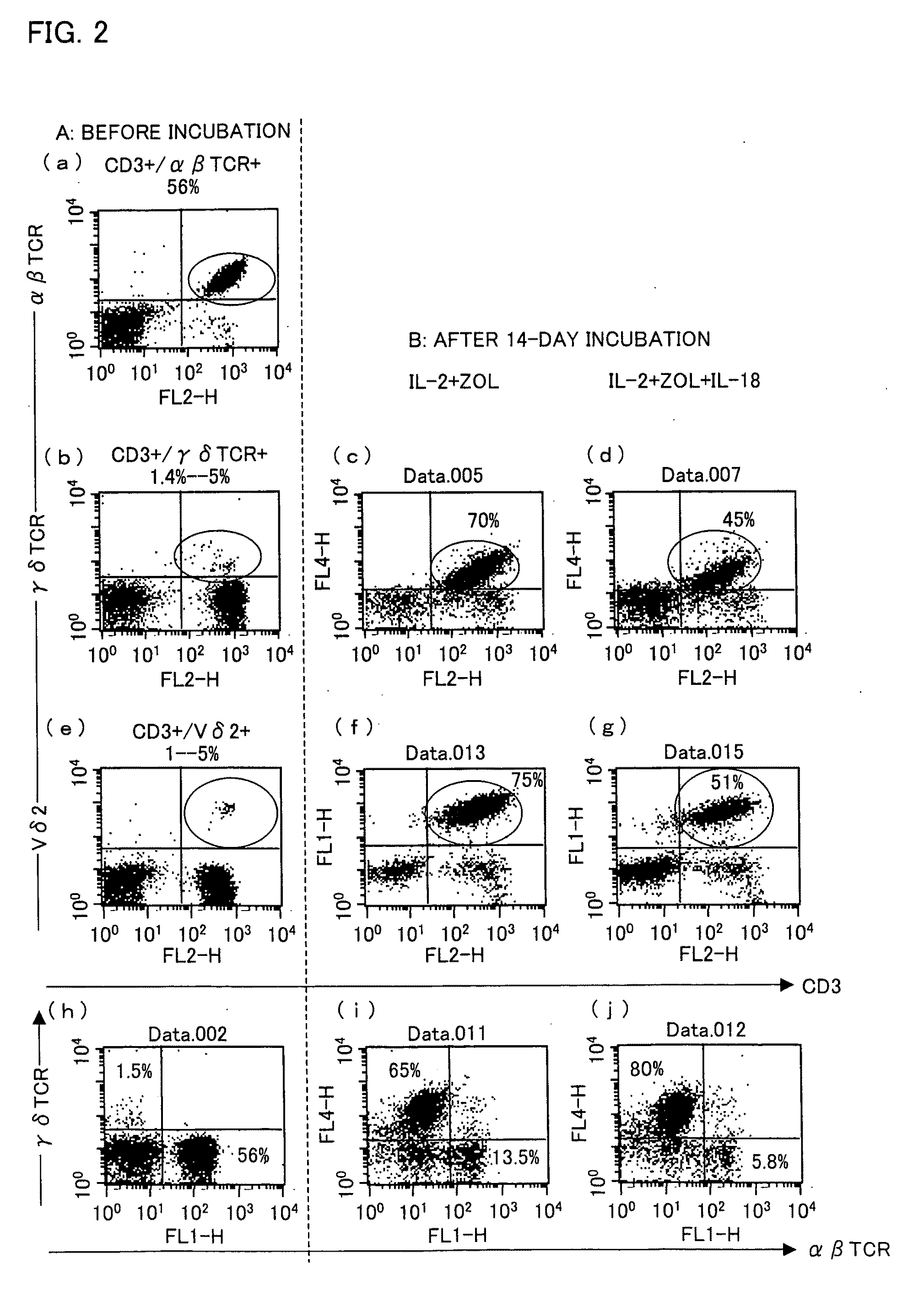
A Vγ9Vδ2 T cell proliferation agent includes at least a bisphosphonate, interleukin 2, and interleukin 18. Since IL-18 has properties that improve cell viability by inhibiting cell death, IL-18 is presumably capable of acting as a cofactor for the bisphosphonate so as to significantly increase the effect of Vγ9Vδ2 T cell proliferation by the bisphosphonate and the IL-2. This allows providing a Vγ9Vδ2 T cell proliferation agent capable of growing Vγ9Vδ2 T cells with a proliferated efficiency significantly high compared to conventional methods so that the proliferated Vγ9Vδ2 T cells have a high antitumor activity and high cytokine productivity.
Owner:HYOGO COLLEGE OF MEDICINE
Medium composition for culturing self-activated lymphocytes and method for culturing self-activated lymphocytes using same
InactiveUS20130157364A1Raise the ratioLess side effectsCancer antigen ingredientsImmunoglobulinsNatural Killer Cell Inhibitory ReceptorsCD16
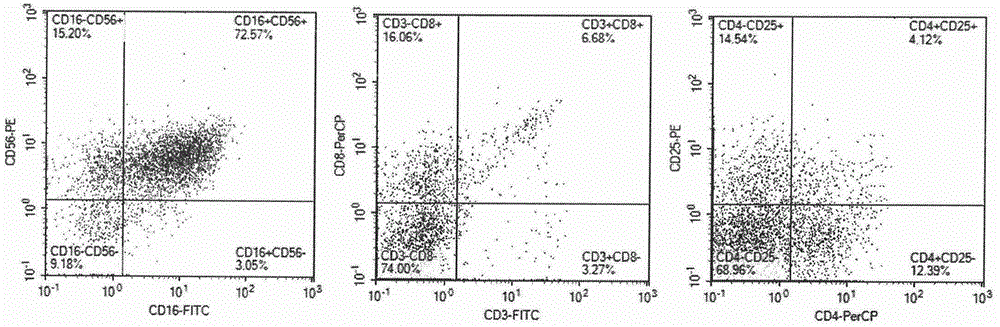
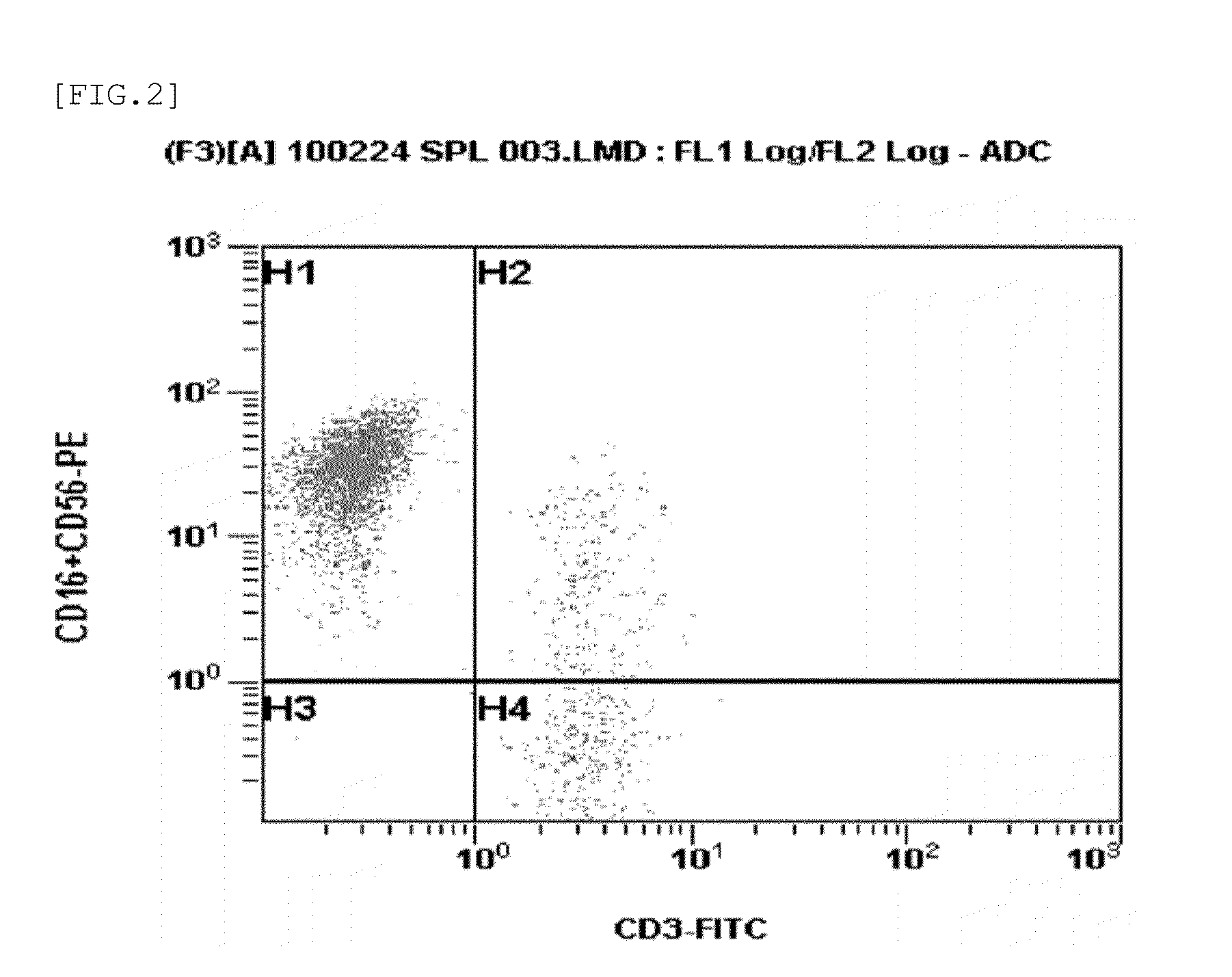
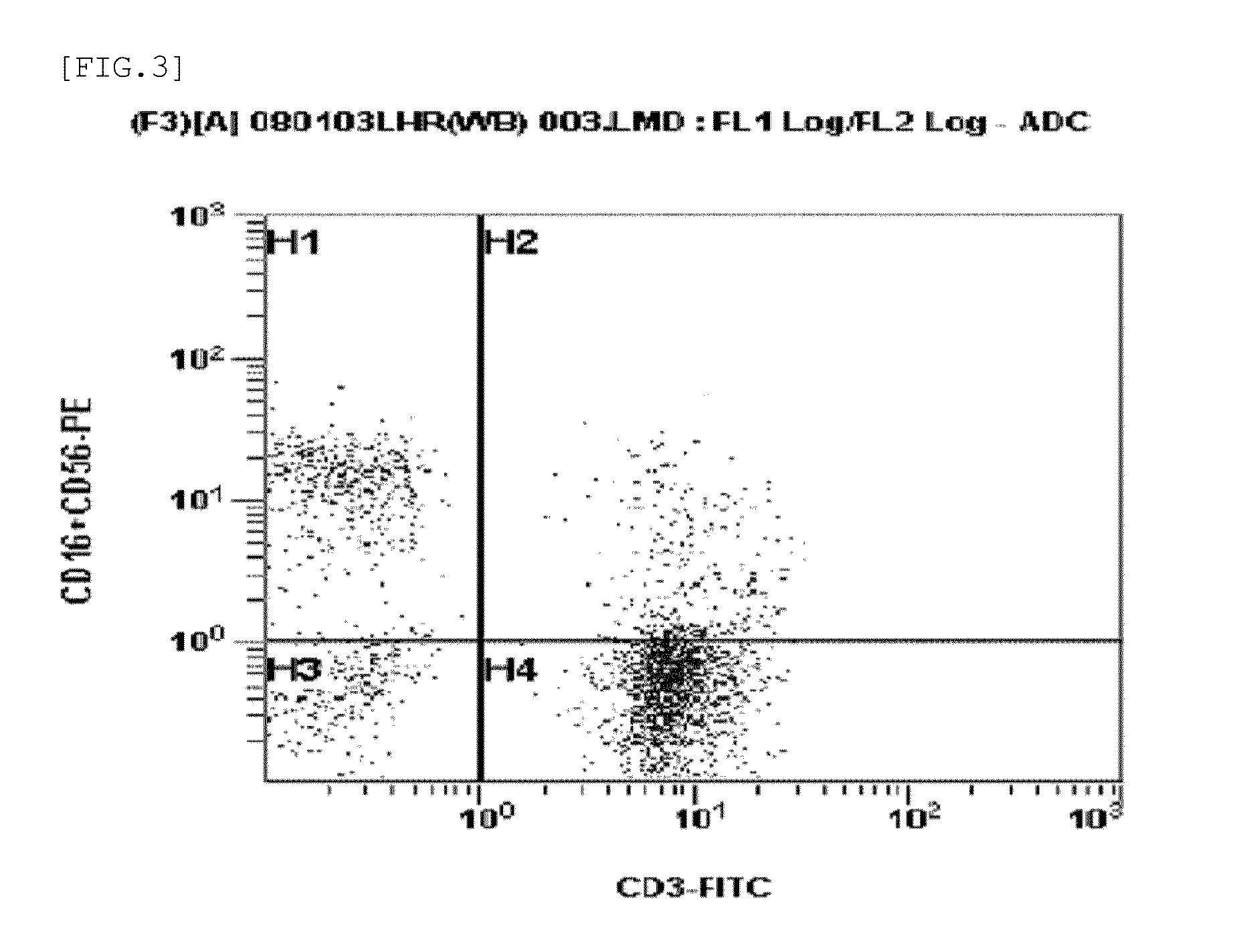
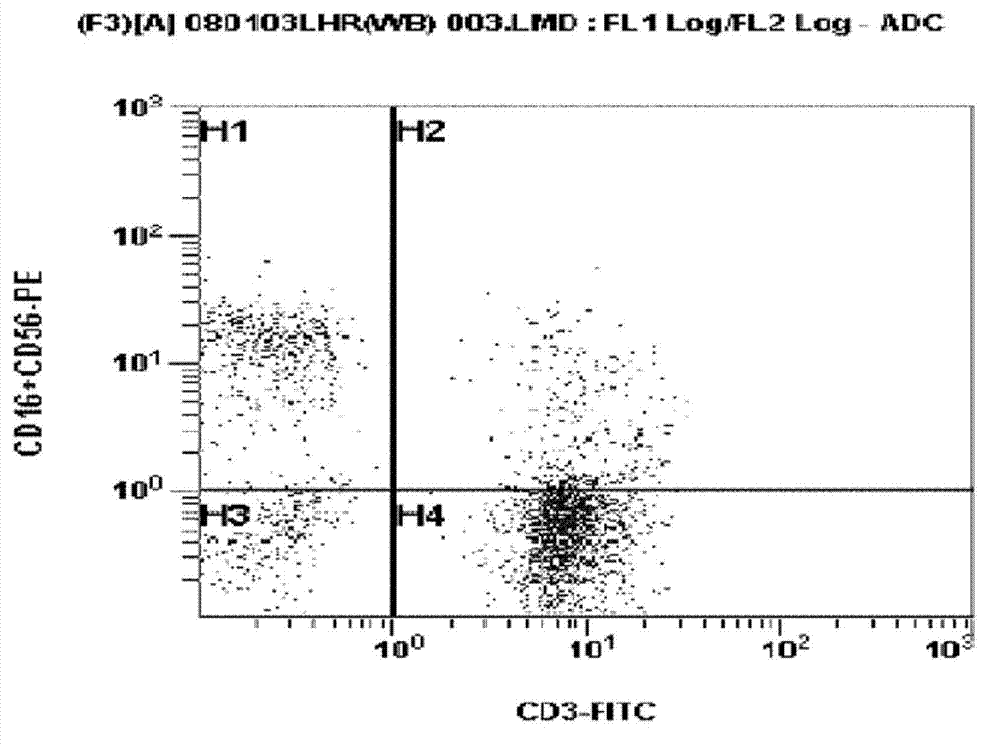
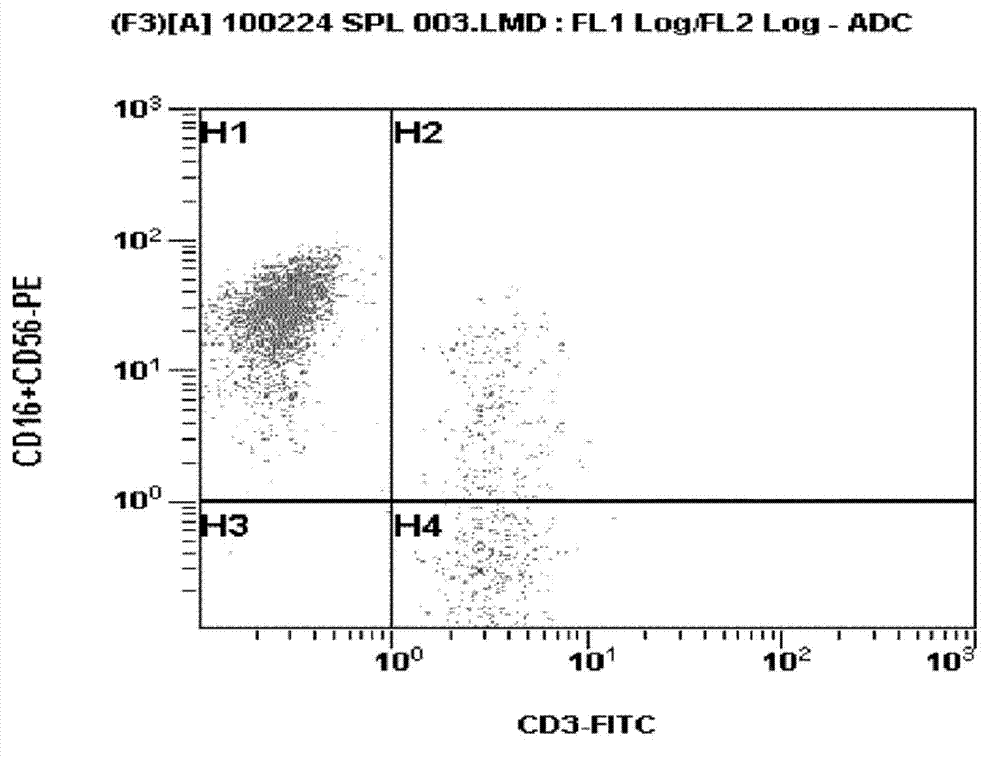
Disclosed is a medium composition for culturing self-activated lymphocytes, which contains anti-CD3 antibody and anti-CD16 antibody in addition to interleukin 2 (IL-2), interleukin 12 (IL-12) and interleukin 18 (IL-18) in a medium, and thus can efficiently proliferate and activate NK cells, T cells and NKT cells and, at the same time, can significantly increase the ratio of NK cells in lymphocytes so as to provide immunocytes having excellent effects on the treatment of various kinds of malignant tumors, and a method for culturing self-activated lymphocytes using the medium composition.
Owner:CELLTECH LTD
Medium composition for culturing self-activated lymphocytes and method for culturing self-activated lymphocytes using same
InactiveCN103080302ARaise the ratioLittle side effectsImmunoglobulins against cell receptors/antigens/surface-determinantsCancer antigen ingredientsCD16Interleukin II
Disclosed is a medium composition for culturing self-activated lymphocytes, which contains anti-CD3 antibody and anti-CD16 antibody in addition to interleukin 2 (IL-2), interleukin 12 (IL-12) and interleukin 18 (IL-18) in a medium, and thus can efficiently proliferate and activate NK cells, T cells and NKT cells and, at the same time, can significantly increase the ratio of NK cells in lymphocytes so as to provide immunocytes having excellent effects on the treatment of various kinds of malignant tumors, and a method for culturing self-activated lymphocytes using the medium composition.
Owner:CELLS SCI CORP
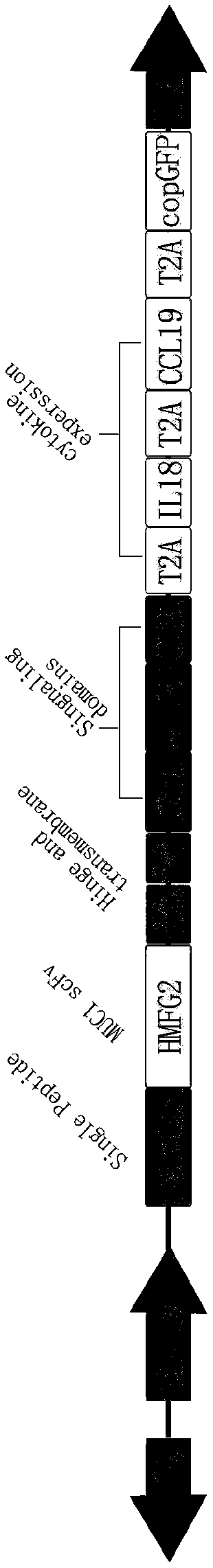
Preparation method of CAR (Cheimeric Antigen Receptors)-T cells for co-expressing IL18 (Interleukin 18) and CCL19 (Chemokine C-C motif ligand 19) protein and targeting MUC1 gene
InactiveCN109055430ADisinhibitionImprove permeabilityAntibody mimetics/scaffoldsNGF/TNF-superfamilyAntigen receptorsT cell
The invention relates to a preparation method of CAR (Cheimeric Antigen Receptors)-T cells for co-expressing IL18 (Interleukin 18) and CCL19 (Chemokine C-C motif ligand 19) protein and a targeting MUC1 gene. The preparation method comprises the following steps: transfecting DC<4+> / CD<+8> positive cells by lentivirus containing IL18 and CCL19 protein coding sequences and targeting MUC1 CAR; screening and culturing amplified and transfected T cells to obtain the CAR-T cells for co-expressing IL18 and CCL19 protein and the targeting MUC1 gene. According to the preparation method provided by the invention, the proliferation capability and the life cycle of the CAR-T cells are enhanced through expressing third cell signals IL18 and CCL19, improving the osmosis effect of the CAR-T cells in solidtumors and maintaining the killing effect of the CAR-T cells on the solid tumors. Furthermore, the co-expressed IL18 and CCL19 are connected through a 2A short peptide and can be expressed respectively.
Owner:杭州荣泽生物科技集团有限公司
Method of treatment using anti-IL-18 antibody
InactiveUS20060110389A1Immunoglobulins against cytokines/lymphokines/interferonsAntibody ingredientsAutoimmune conditionAutoimmune disease
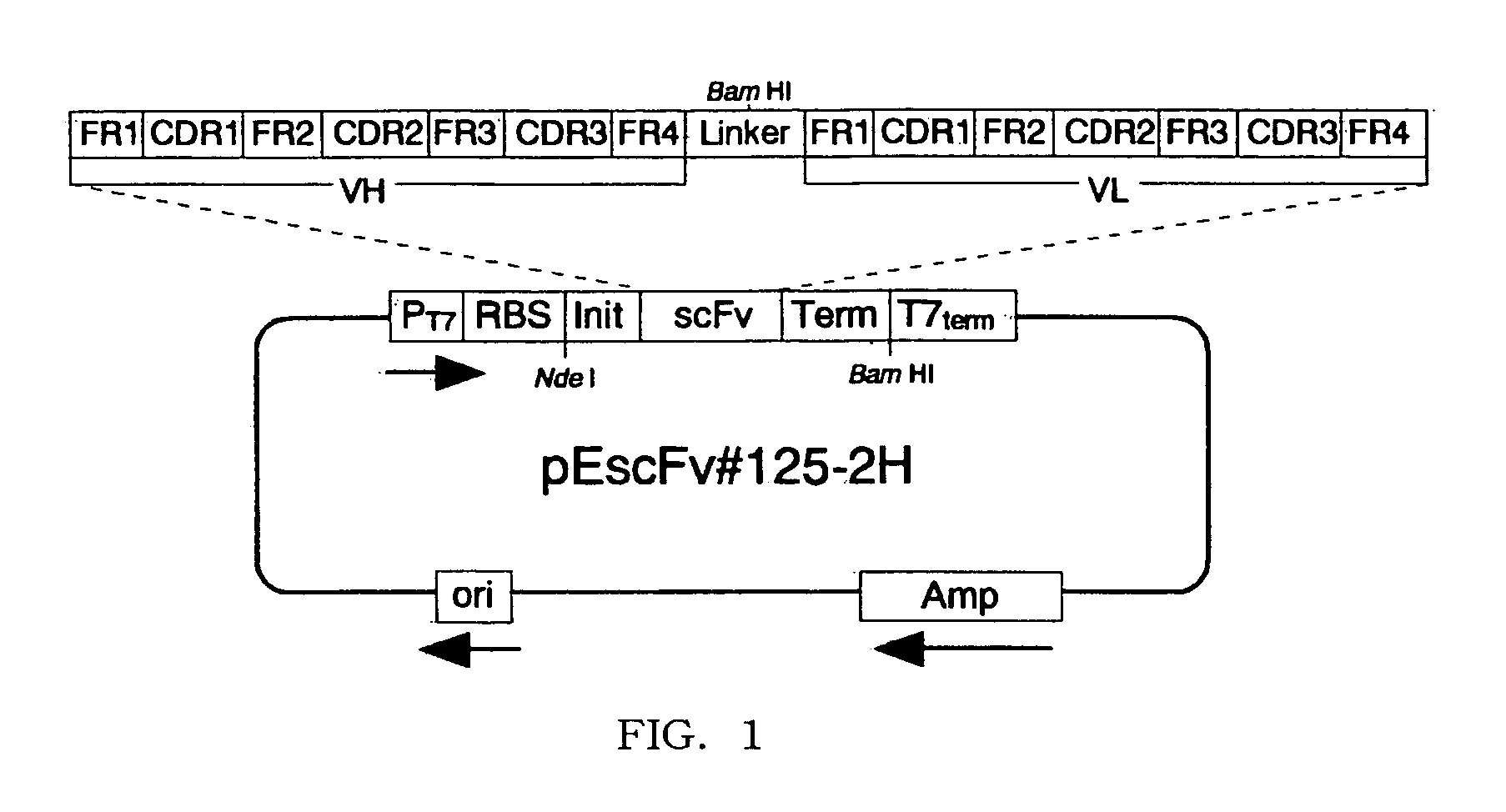
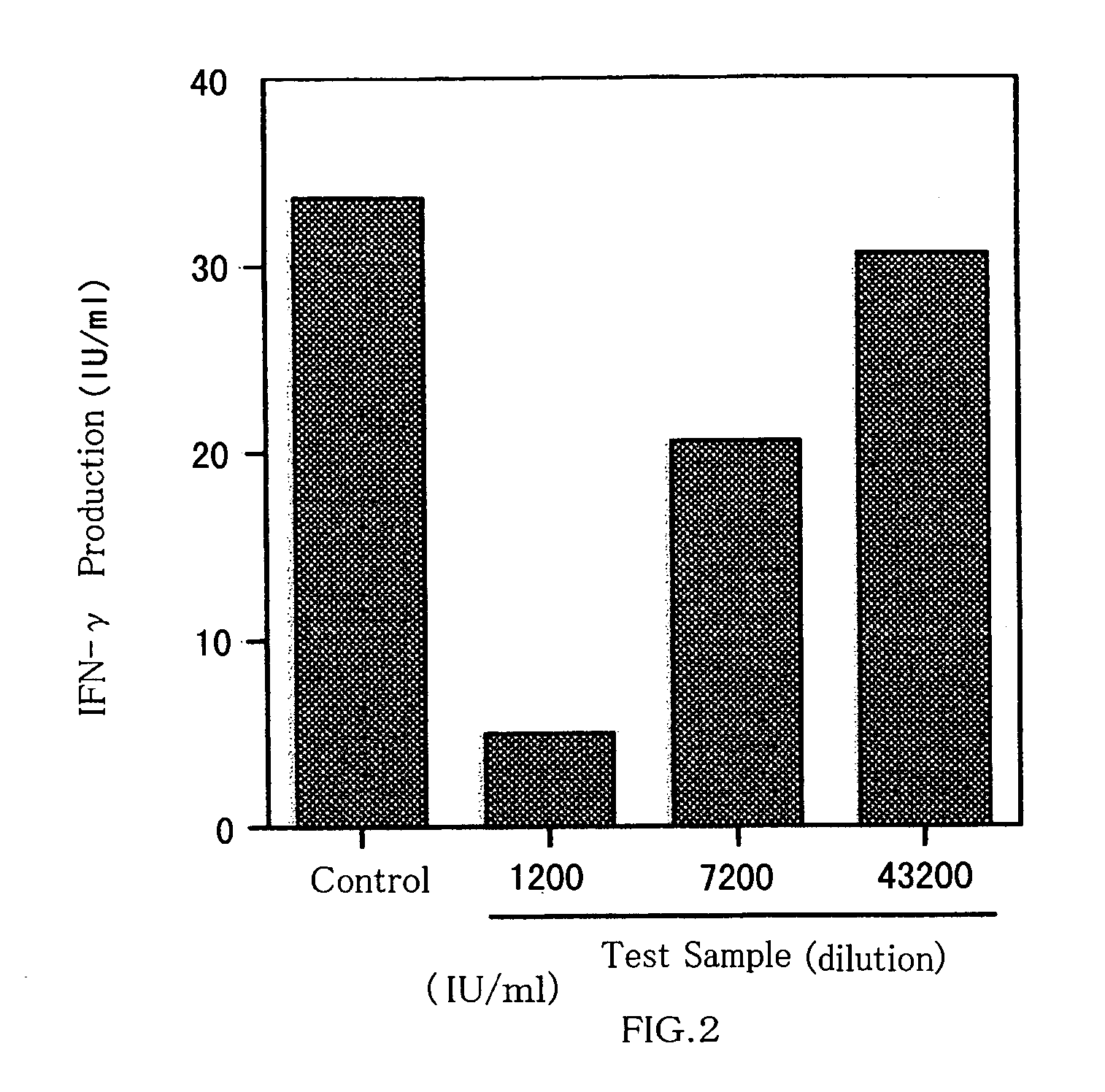
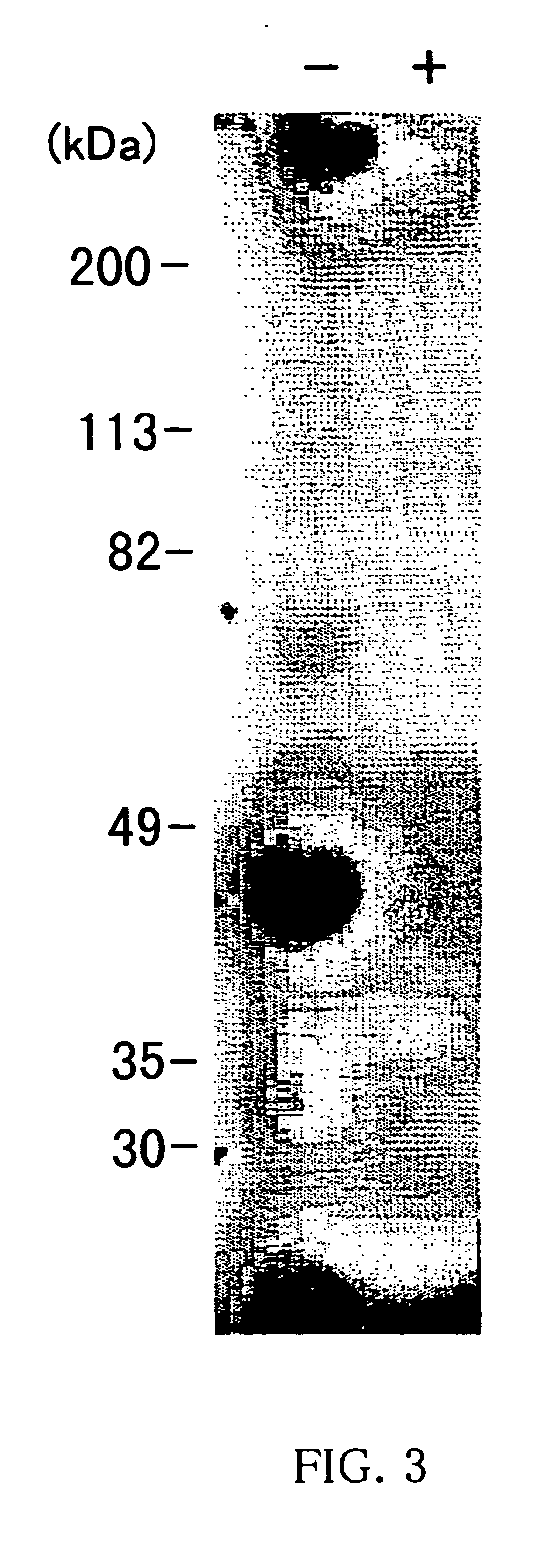
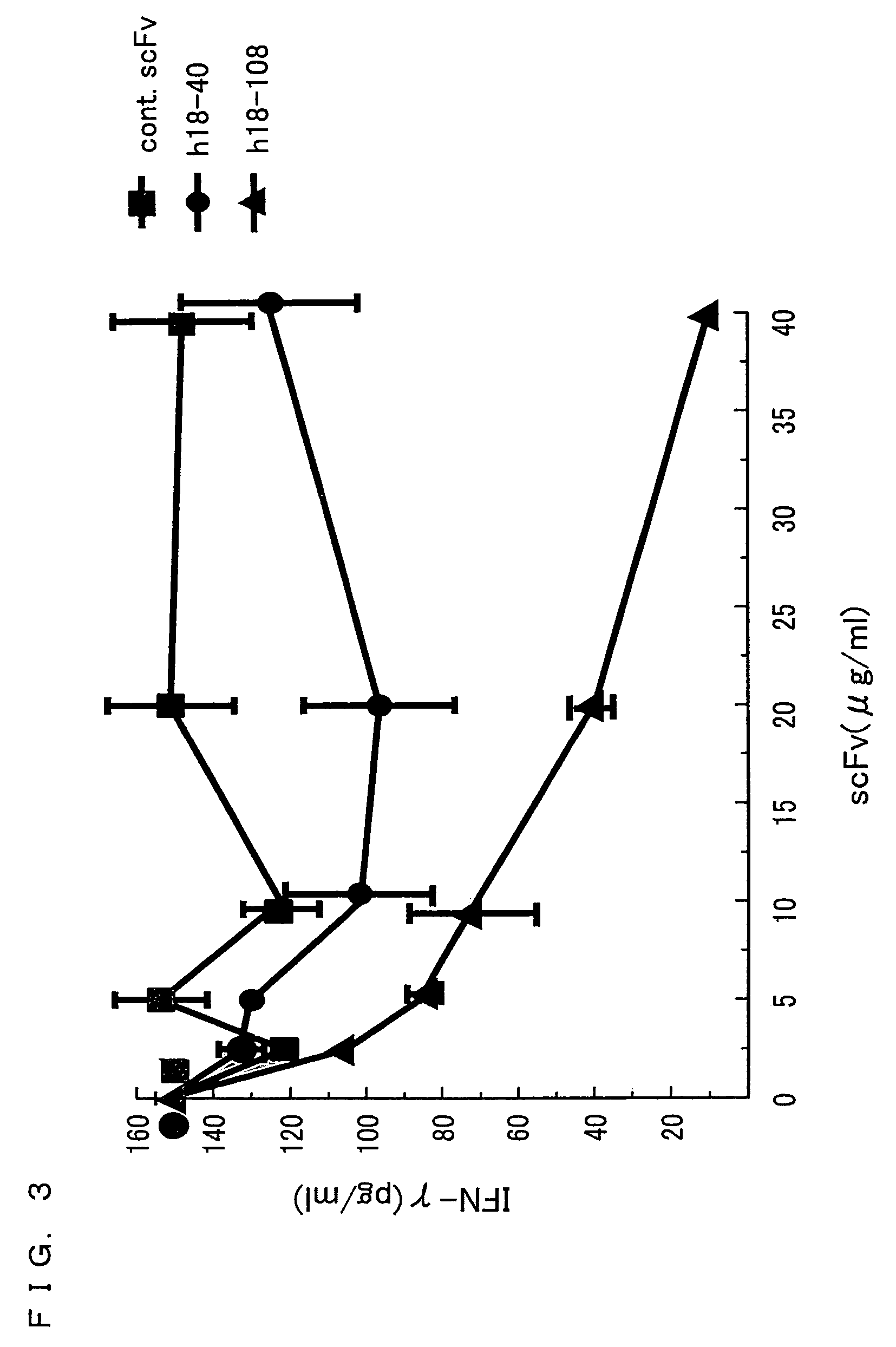
Disclosed are artificially produced peptide capable of neutralizing the biological activities of IL-18, which comprises a part or the whole of the variable regions in anti interleukin 18 antibody, including single chain variable region fragments and humanized antibodies, a process of producing the peptide, and uses thereof. The peptide is useful as pharmaceutical to treat and prevent diseases such as autoimmune diseases and inflammatory diseases, where the biological activities of interleukin-18 are involved.
Owner:HAYASHIBARA BIOCHEMICAL LAB INC
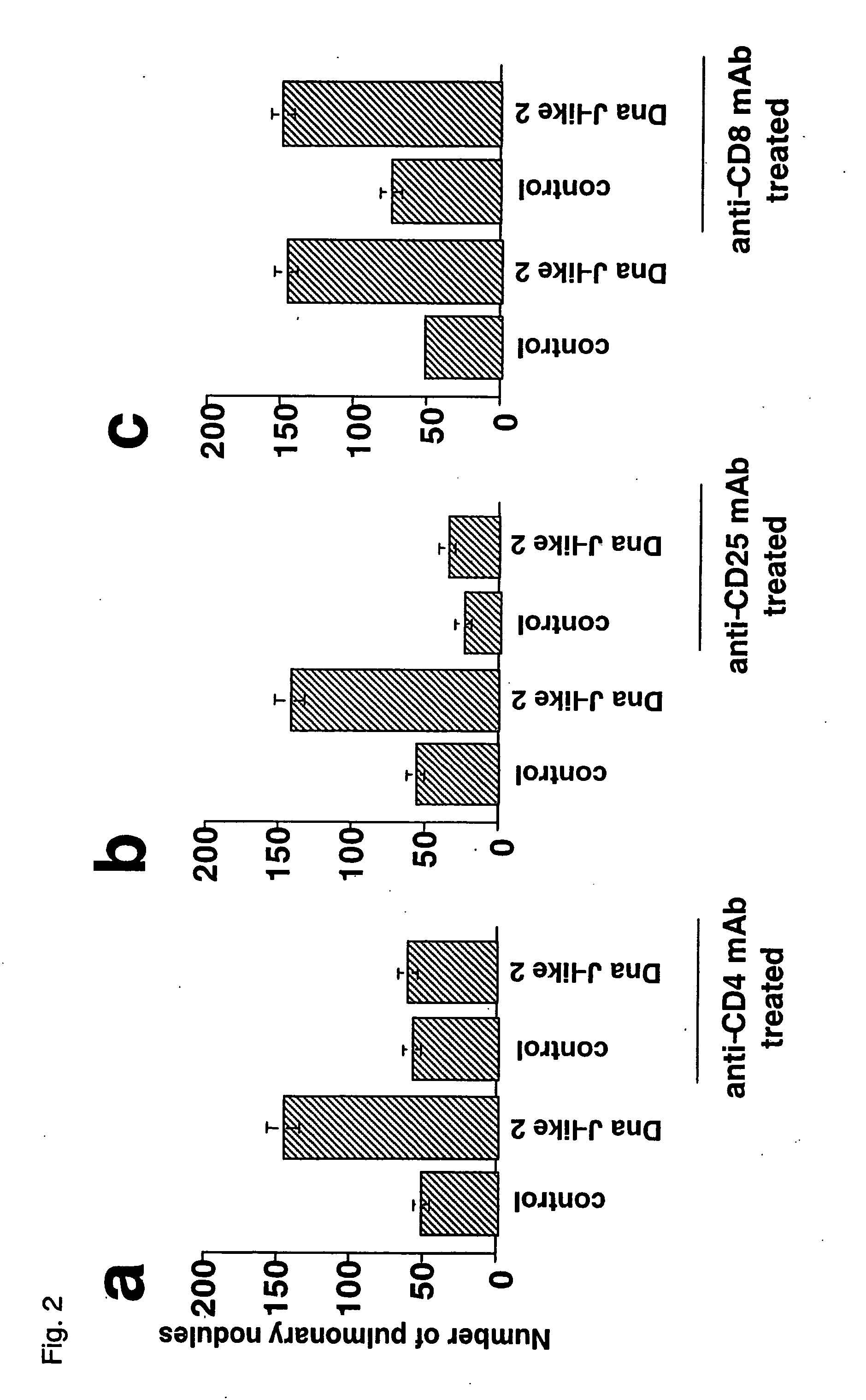
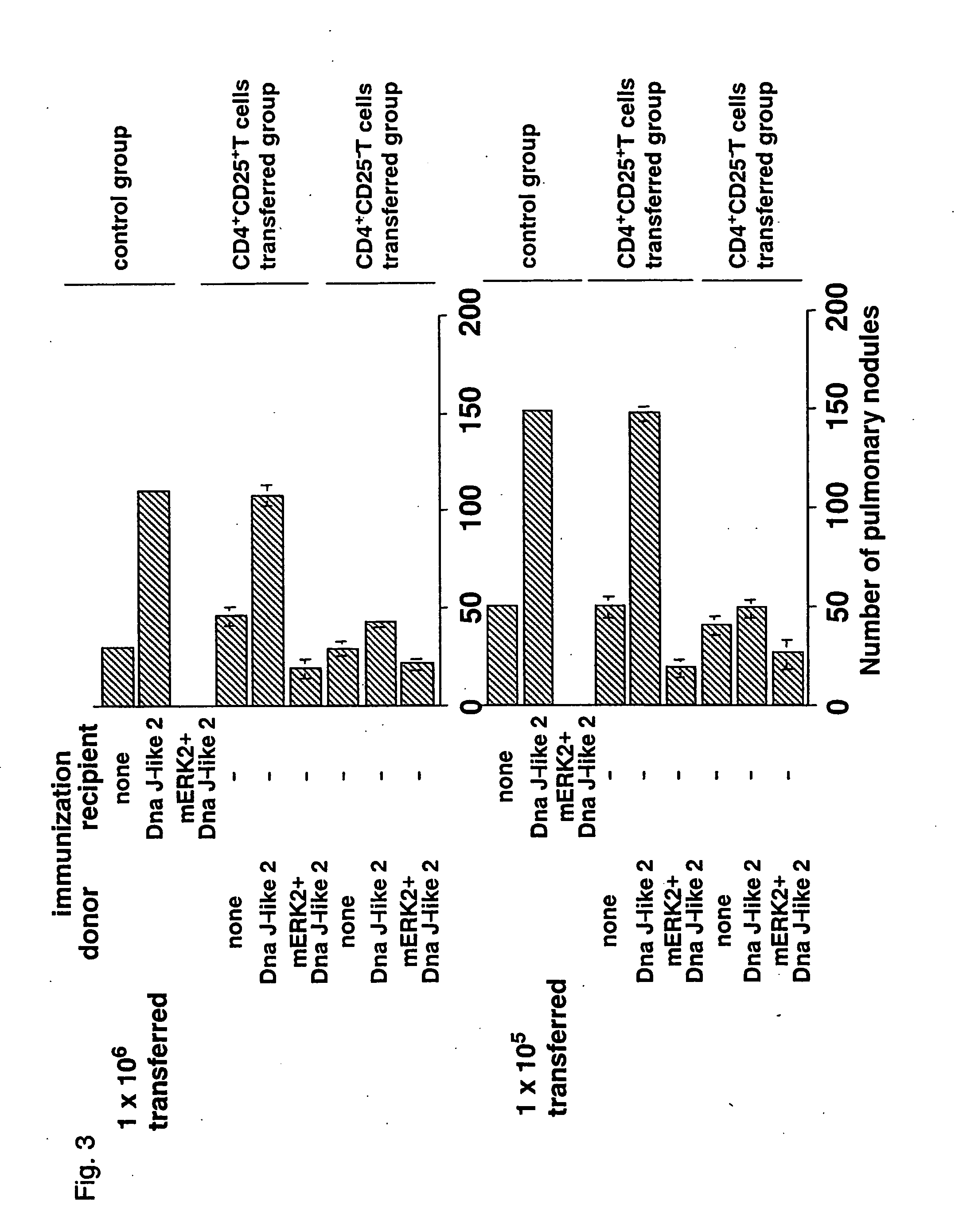
Method and composition for regulating the activity of regulatory t cells
InactiveUS20060121029A1High activitySuppression of rejection and graft-versus-host reactionsPeptide/protein ingredientsGenetic material ingredientsGraft versus host reactionsAntigen
The present invention provides a composition and method of regulating the activity of regulatory T cells. The present invention relates to a composition containing an antigen recognized by CD4+CD25+ regulatory T cells or an expression vector encoding such an antigen and a method of controlling an immune response from a mammal by administrating the composition to the mammal. Furthermore, the present invention provides effective means for suppressing a rejection reaction and a graft-versus-host reaction in transplantation and for prevention and treatment of an autoimmune disease or an allergic disease. Furthermore, an immunosuppression condition can be removed by the administration of Interferon-γ or a combination of Interleukin 12 and Interleukin 18. In other words, those cytokine actions and the sensitization with an SEREX antigen are suitably combined to artificially manipulate regulatory T cells, allowing the cells to be applied to an autoimmune disease, reactions accompanied with organ transplantation, allergic reaction, control of tumor immunity, and the like.
Owner:IMMUNOFRONTIER
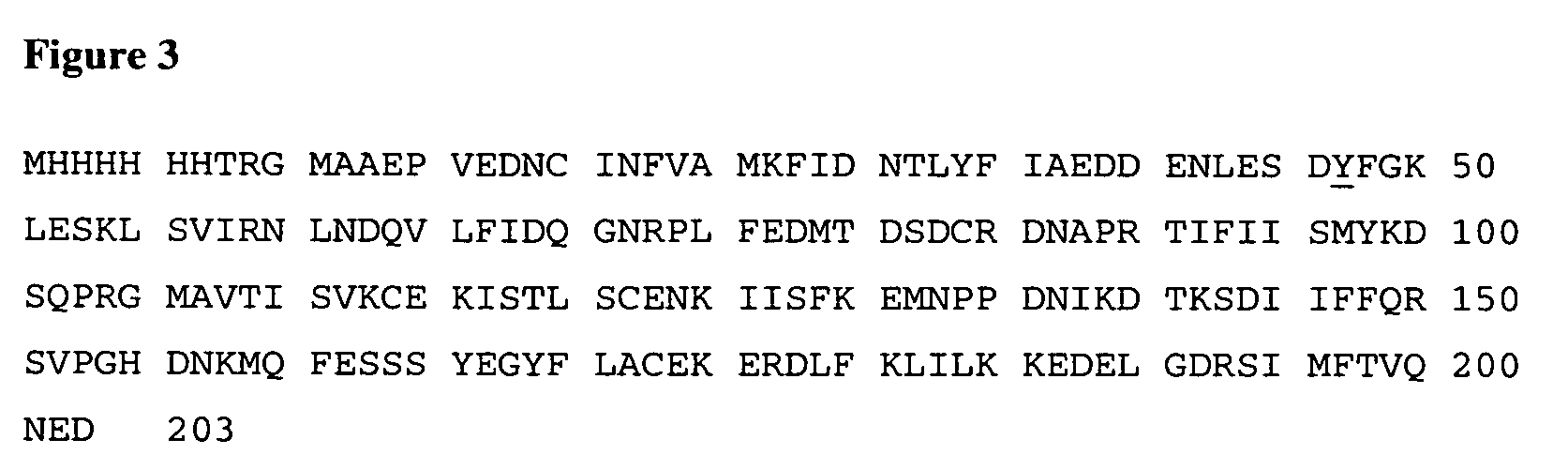
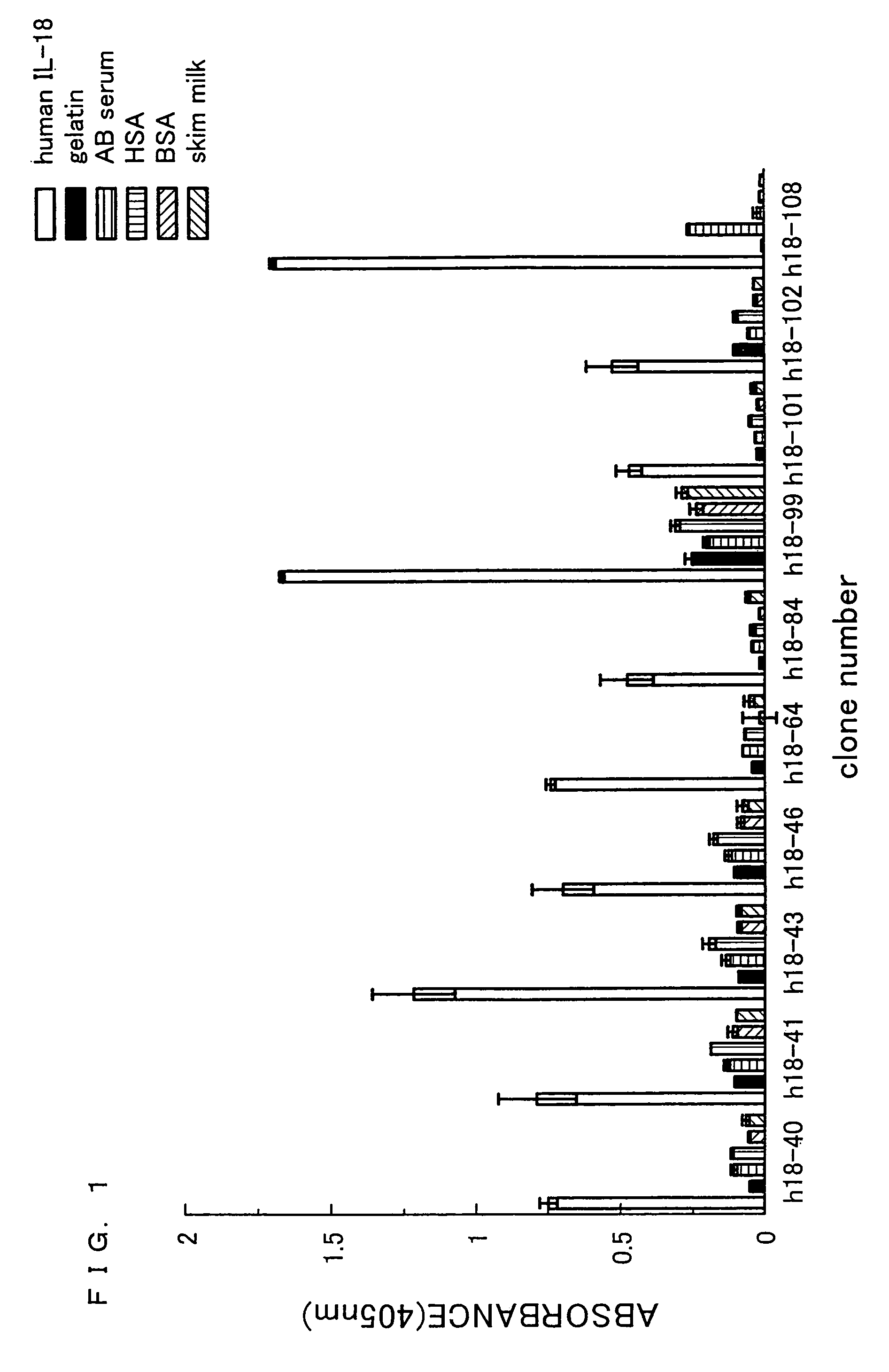
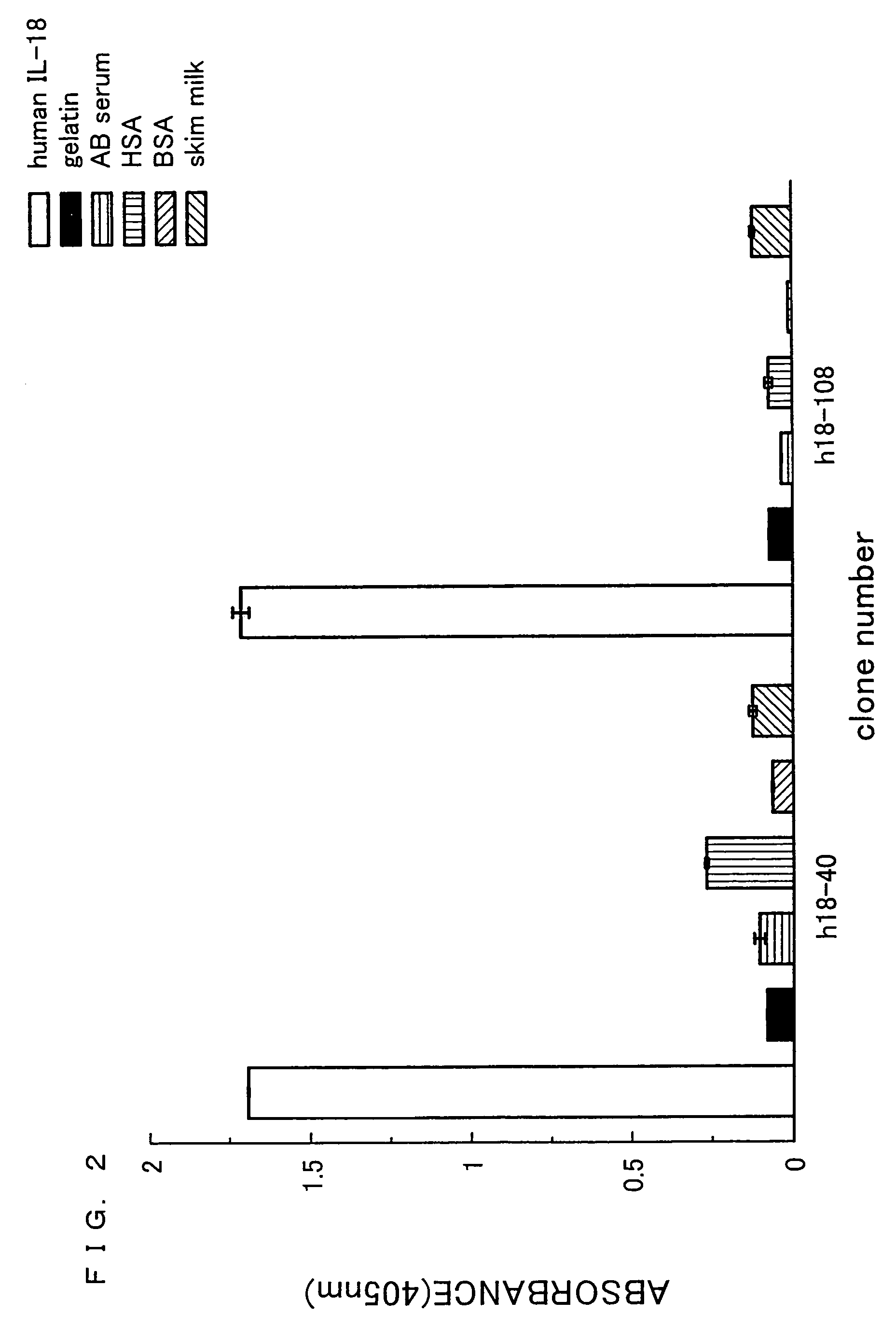
Human anti-human interleukin-18 antibody, fragment thereof and method for using same
InactiveUS7491803B2Significant effectImprove security levelSugar derivativesAntipyreticComplementarity determining regionWhite blood cell
A human-derived human anti-human IL-18 antibody of the present invention is an antibody against human IL-18, the antibody including: an H-chain complementarity determining region consisting of (a) a polypeptide consisting of amino-acid sequences represented by SEQ ID NOS: 4 to 6, or (b) a polypeptide which is a mutant of the polypeptide (a) and which serves as the H-chain complementarity determining region; and an L-chain complementarity determining region against human interleukin-18 consisting of (c) a polypeptide consisting of amino-acid sequences represented by SEQ ID NOS: 10 to 12, or (d) a polypeptide which is a mutant of the polypeptide (c) and which serves as the L-chain complementarity determining region. This makes it possible to provide a human anti-human IL-18 antibody and a method for using the antibody.
Owner:JAPAN SCI & TECH CORP +1
Vgamma9Vdelta2 T cell proliferation agent, method for producing activated Vgamma9Vdelta2 T cells, and uses thereof
InactiveUS20100009447A1Increase in sizeGood effectOrganic active ingredientsPeptide/protein ingredientsWhite blood cellCellular viability
A Vγ9Vδ2 T cell proliferation agent includes at least a bisphosphonate, interleukin 2, and interleukin 18. Since has properties that improve cell viability by inhibiting cell death, IL-18 is presumably capable of acting as a cofactor for the bisphosphonate so as to significantly increase the effect of Vγ9Vδ2 T cell proliferation by the bisphosphonate and the IL-2. This allows providing a Vγ9Vδ2 T cell proliferation agent capable of growing Vγ9Vδ2 T cells with a proliferated efficiency significantly high compared to conventional methods so that the proliferated Vγ9Vδ2 T cells have a high antitumor activity and high cytokine productivity.
Owner:HYOGO COLLEGE OF MEDICINE
Compositions for regulation of tumor necrosis factor-alpha
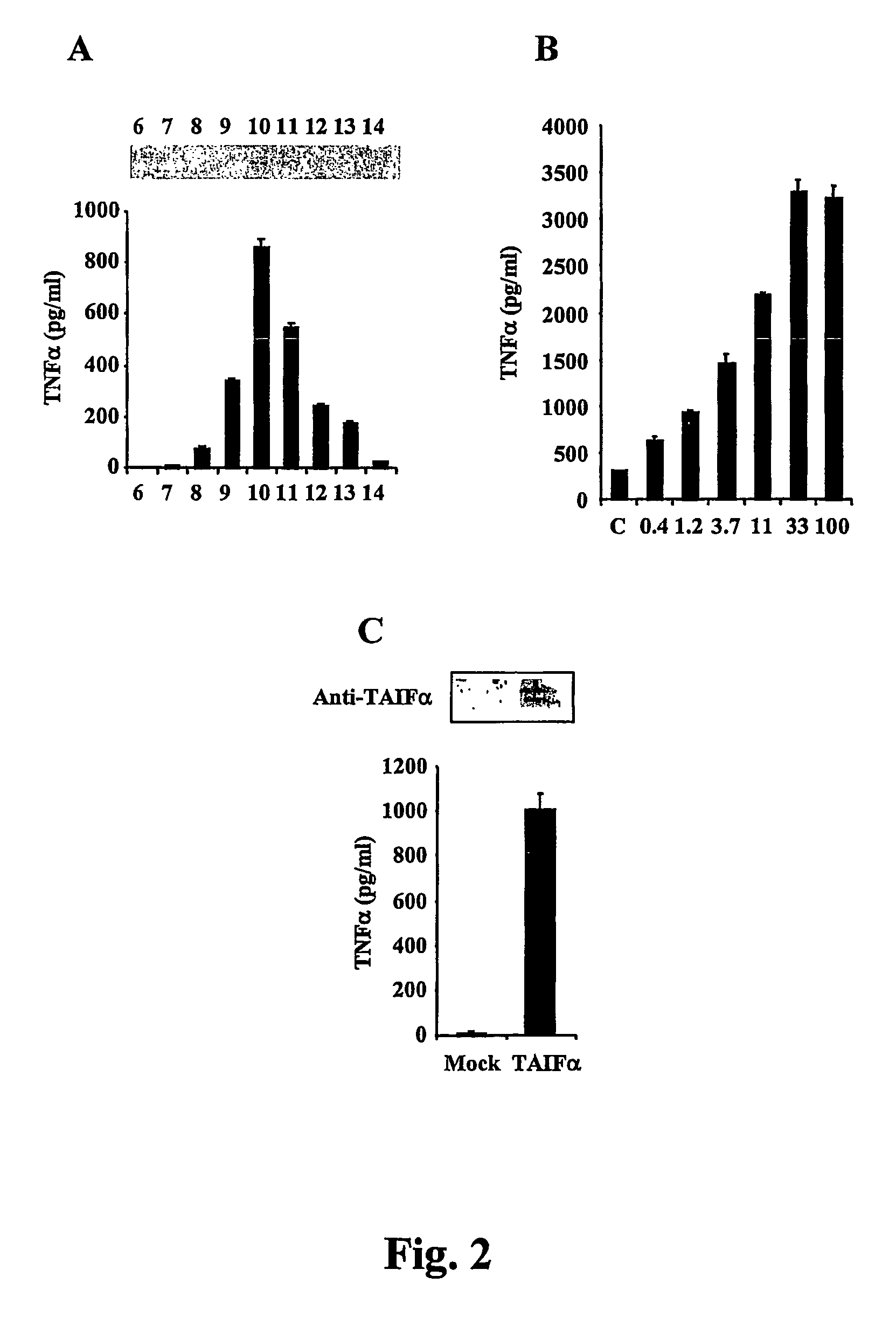
The present invention relates to compositions relating a nucleic acid encoding an interleukin 18-inducible cytokine termed tumor necrosis factor-alpha inducing factor (TAIF) or interleukin-32 (IL-32). In particular, the present invention provides vectors for expressing proteins useful for treating autoimmune diseases and cancer, in part by regulation of tumor necrosis factor-alpha expression.
Owner:UNIV OF COLORADO THE REGENTS OF
Anti-IL-18 antibodies and their uses
ActiveUS9255144B2Reduced activityInhibit bindingAntibody mimetics/scaffoldsAntipyreticAnti-CEA AntibodyChimeric antibody
The present invention provides human, humanized and / or chimeric antibodies as well as fragments, derivatives / conjugates and compositions thereof with a specific binding affinity for interleukin-18. The invention includes the use of these antibodies for diagnosing and treating diseases associated with increased IL-18 activity, the latter in the form of a pharmaceutical composition.
Owner:MEDIMMUNE LTD
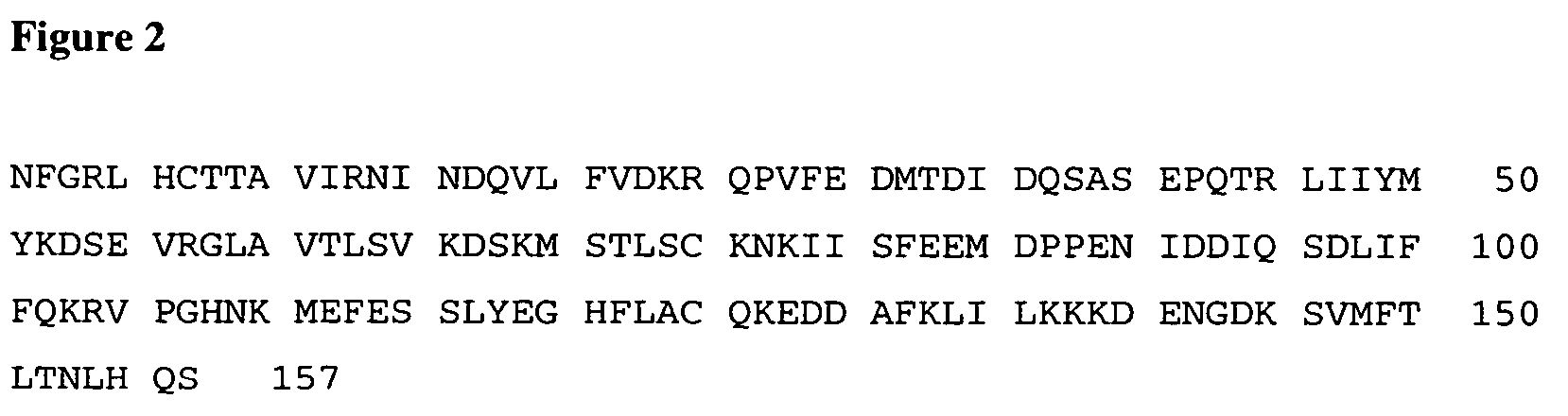
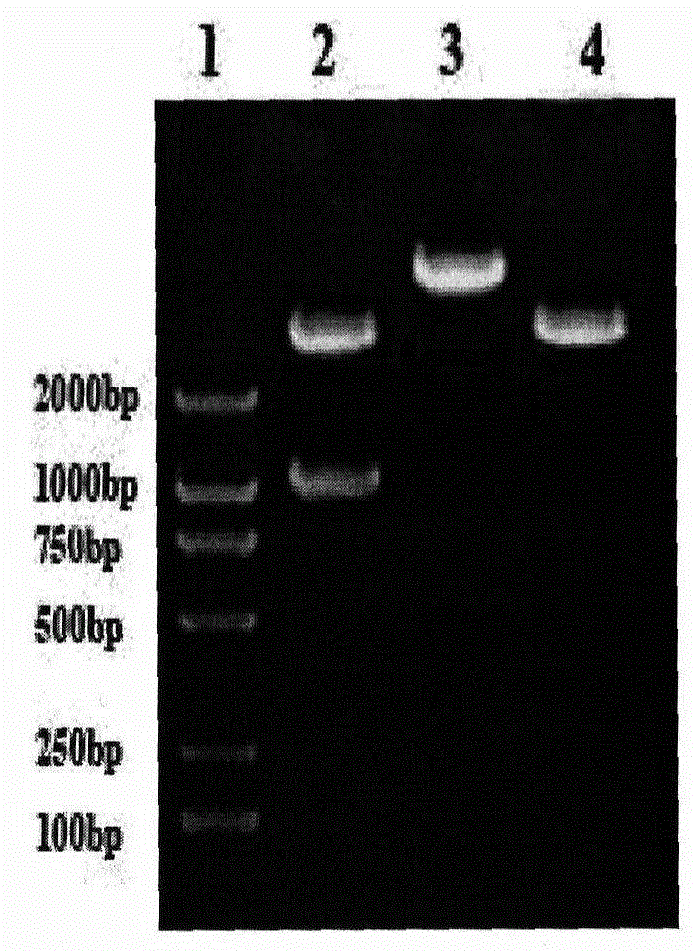
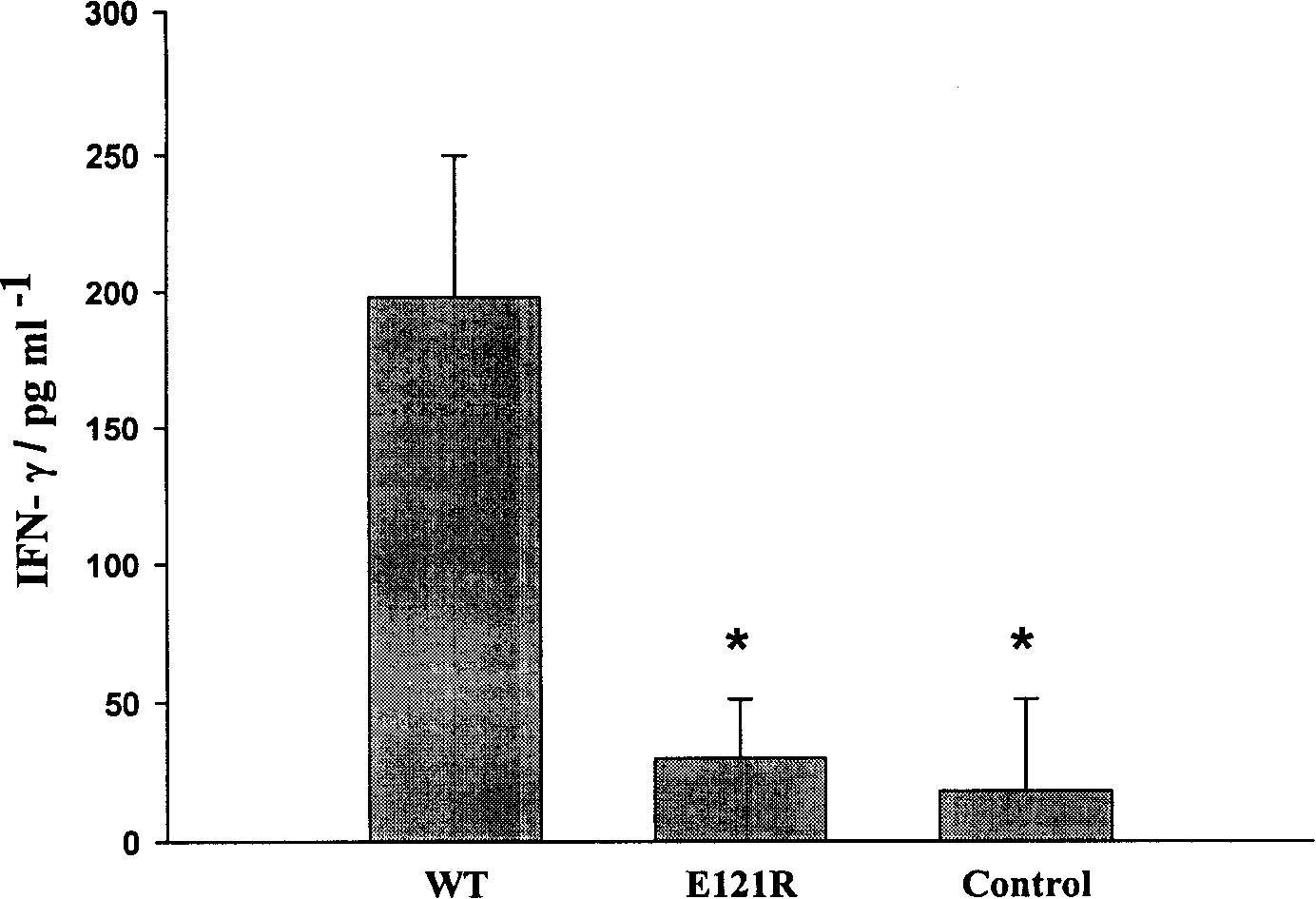
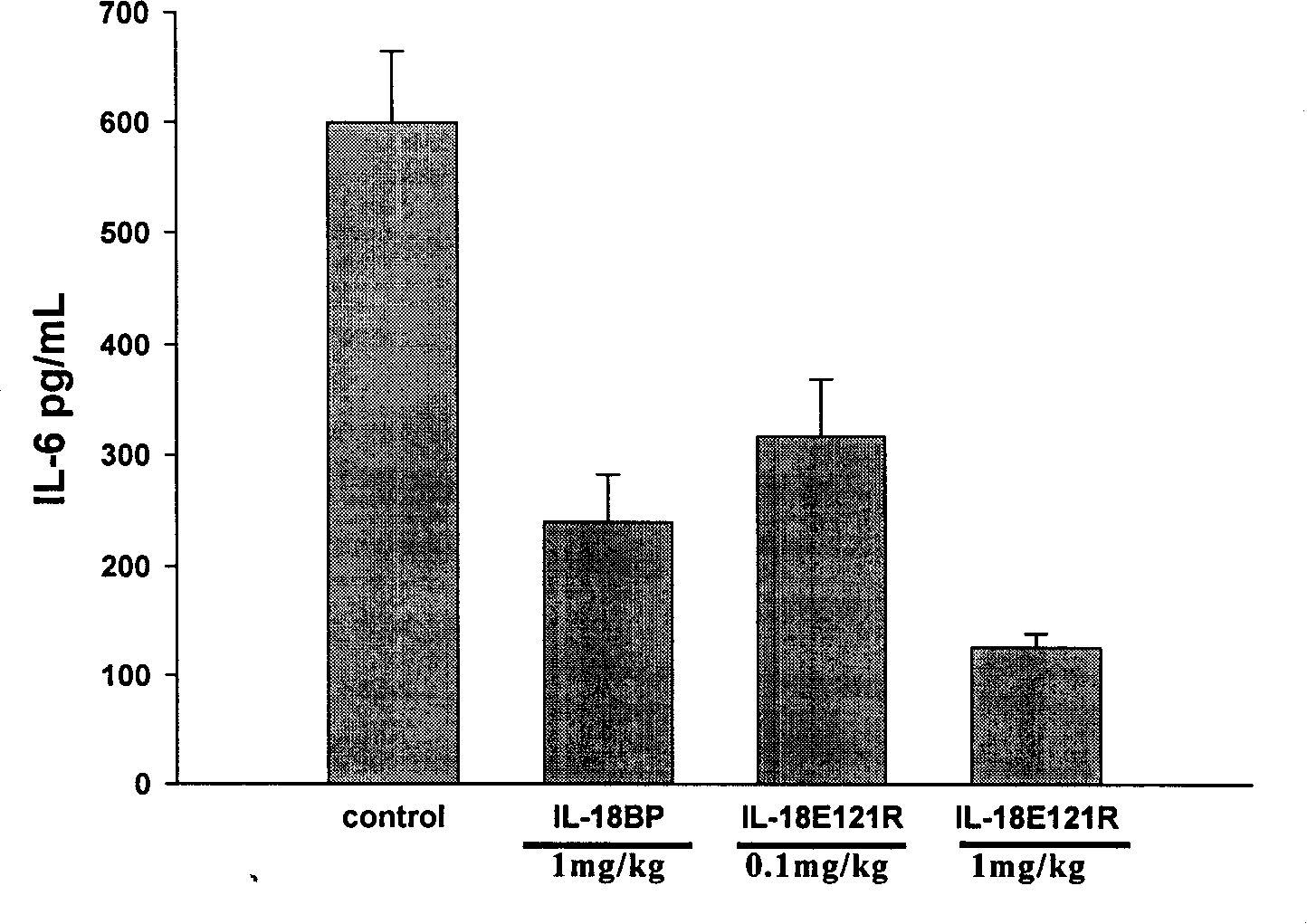
Interleukin-18 mutants, their production and use
The invention provides mutants of IL-18 with lower affinity to IL-18BP than the wild type IL-18 molecule.
Owner:ARES TRADING SA
Formulations for Nutritional Support in Subjects in Need Thereof
InactiveUS20190388518A1Growth inhibitionAvoid problemsHydrolysed protein ingredientsDigestive systemGut immunityBacteroides
Formulations having a protein component, in which the protein contains one or more digestion-aiding proteins, and one or more immunoprotective proteins. The ratio by weight of the one or digestion-aiding proteins to the one or more immunoprotective proteins may be about 12:1 to about 1:1. The formulations may also contain a fat component, a carbohydrate component, and vitamins and minerals. These formulations can be used to provide nutritional support to a subject, either as dietary supplements or as a primary source of nutrition, such as for an infant formula. The formulations may also be used to promote or induce proliferation of intestinal cells, promote or induce differentiation of intestinal cells, prevent or inhibit growth of enteropathogenic Escherichia coli in the digestive system of a subject, prevent or inhibit bacterial growth in the intestinal lumen, increase interleukin-18 secretion by intestinal cells, or increase intestinal immunity.
Owner:SECOND SCI INC
Use of interleukin-18 inhibitors to inhibit tumor metastasis
Owner:ARES TRADING SA
Construction expression of fusion gene carrier and its application
InactiveCN1757736ALow costLower medical costsPeptide/protein ingredientsAntineoplastic agentsGackstroemiaReverse transcriptase
A human telomerase reverse-transcriptase (hTERT) / human interleukin 18 (hIL 18) fusion protein with composite function is prepared from 2 cell factors with similar or complementary functions through artificial reforming of their linking terminals and configuring. The expression of its carrier is created for increasing its target killing power to tumor cells and the dendritic cell mediated immune-effect. It can be used to prepare the dendritic cell vaccine for treating cancer.
Owner:ZHEJIANG UNIV
Method of treating cancer by administering conjugates comprising human il-18 and substitution mutants thereof
ActiveUS20080206189A1Peptide/protein ingredientsPharmaceutical non-active ingredientsBiological propertyWater soluble
Human interleukin-18 (IL-18) polypeptides and substitution mutants thereof were conjugated to water-soluble polymers at specific sites on the human IL-18 protein. These conjugated human IL-18 and substitution mutants thereof retain biological activity. These conjugated cytokines demonstrate enhanced and unexpected biological properties when compared to the corresponding unconjugated cytokines.
Owner:GLAXO SMITHKLINE LLC
Detection kit for interleukin-18 (IL-18)
The invention discloses a detection kit for IL-18. The detection kit comprises seven storage assemblies which are respectively used for storing an IL-18 calibrator, an IL-18 control material, an enzyme conjugate working solution, a magnetic bead working solution, a rinsing solution, a substrate solution and a pretreatment reagent, wherein the magnetic bead working solution comprises carboxyl magnetic beads labeled by an IL-18 antibody, and the enzyme conjugate working solution comprises an alkaline phosphatase-labeled anti-IL-18 antibody. The invention also discloses a detection method for detecting IL-18. The detection kit disclosed in the invention has a minimum detection limit of 5 pg / ml, a linear range of 5-5000 pg / ml, high detection sensitivity and a wide linear range, enables detection time to be shortened to 15 min and simplifies detection steps.
Owner:NANTONG EGENS BIOTECH CO LTD
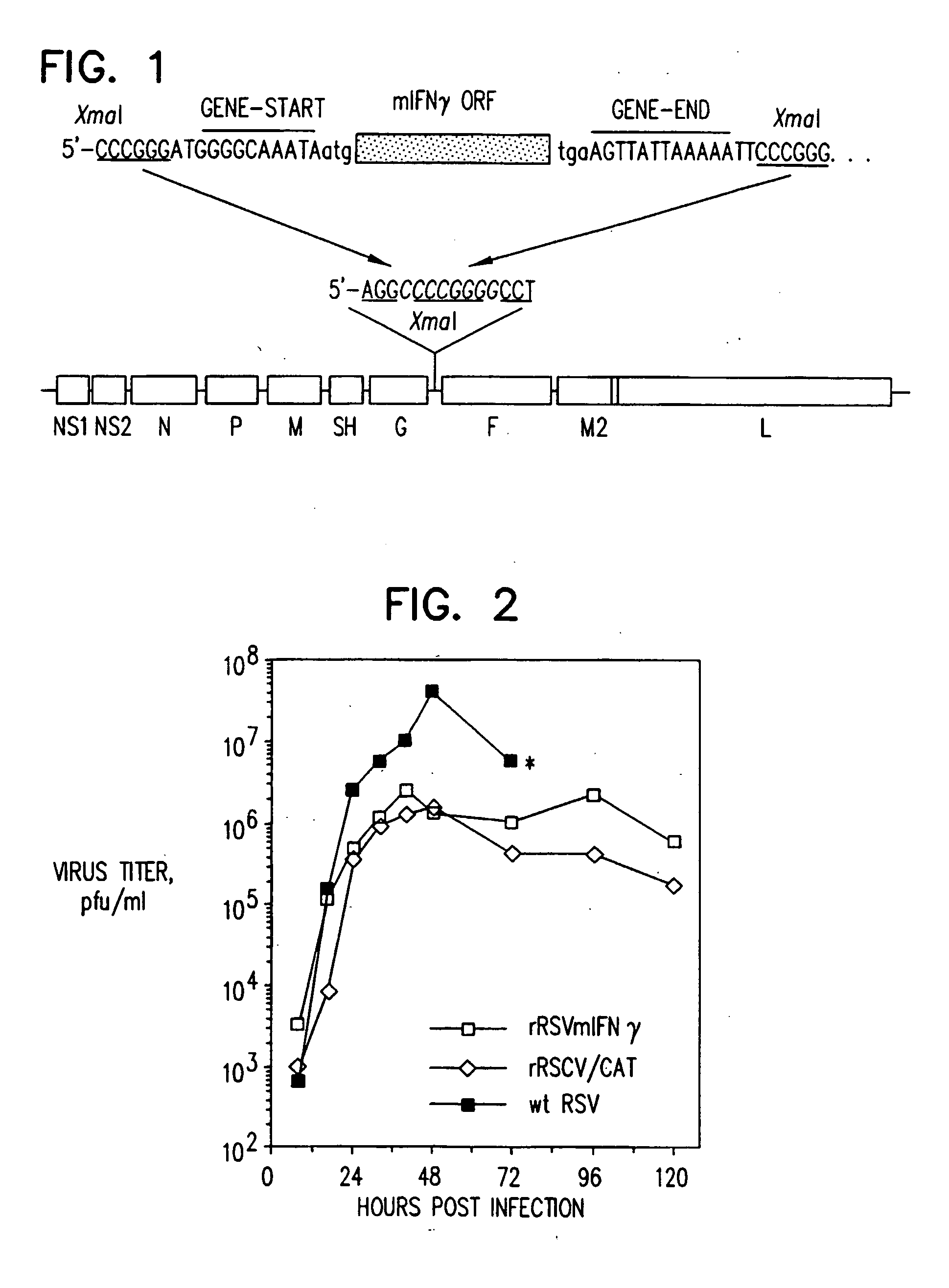
Production of recombinant respiratory syncytial viruses expressing immune modulatory molecules
InactiveUS20050220767A1Altered biological propertyImprove replication efficiencySsRNA viruses negative-senseBiocideInterleukin 6Interleukin 5
Recombinant respiratory syncytial virus (RSV) are provided which express one or more immune modulatory molecules. The recombinant virus is modified by addition or substitution of a polynucleotide sequence encoding the immune modulatory molecule, which is preferably a cytokine. Introduction of the cytokine increase, decrease, or otherwise enhances aspects of viral biology and / or host immune responses to RSV to facilitate vaccine use of the virus. Cytokines for use within the invention include but are not limited to interleukin 2 (IL-2), interleukin 4 (IL-4), interleukin 5 (IL-5), interleukin 6 (IL-6), or interleukin 18 (IL-18), tumor necrosis factor (TNF) alpha, interferon gamma (IFN), and granulocyte-macrophage colony stimulating factor (GM-CSF). The polynucleotide or immune modulatory molecule is preferably added or substituted into the recombinant viral genome or antigenome, typically at an intergenic or other non-coding site, as a separate gene but may be otherwise expressed, for example as a fusion protein.
Owner:COLLINS PETER +3
Recombinant H9N2 subtype avian influenza enhanced multi-epitope vaccine
The invention relates to preparation and application of a recombinant H9N2 subtype avian influenza (Avian influenza H9N2) enhanced multi-epitope vaccine. The vaccine takes neutralizing epitope, Th epitope, CTL epitope and B cell epitope of the major structural protein hemagglutinin (HA), neuraminidase (NA), nucleocapsid protein (NP) and matrix protein 2 (M2) of H9N2 subtype avian influenza virus as the frame structure, after being in flexible linker connection and series connection with cell factor interleukin 18 (chIL-18), the frame structure is then cloned into a pRSETB carrier to be transformed into escherichia coli, and then processes like fermentation, purification and emulsification are carried out, so that an avian influenza enhanced multi-epitope vaccine with ideal immunogenicity is achieved. Animal experiments indicate that the recombinant H9N2 subtype avian influenza enhanced multi-epitope vaccine is not only good in safety, but also can activate effective humoral immune and cellular immune responses.
Owner:GUANGZHOU PUTAI BIOTECH
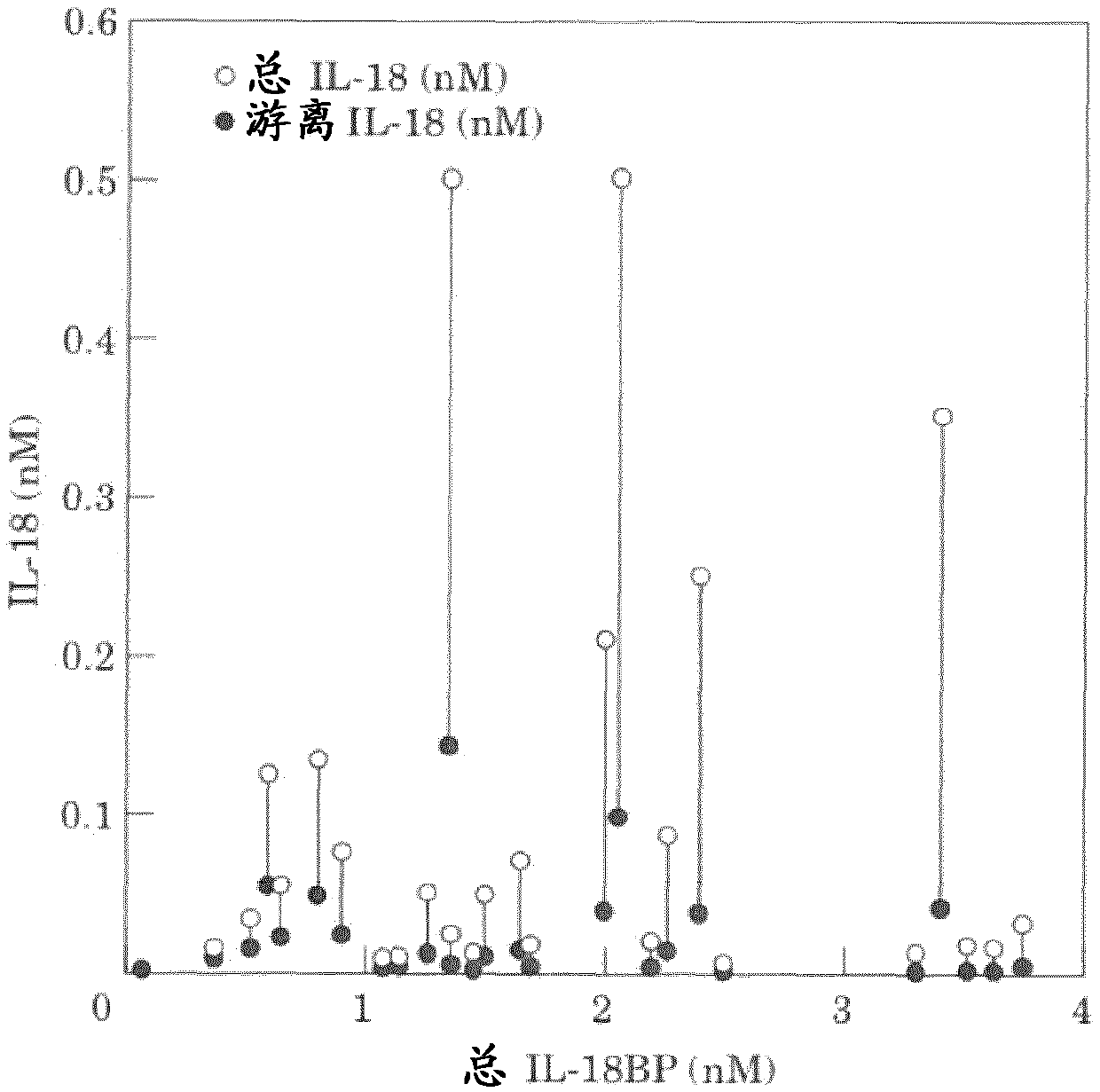
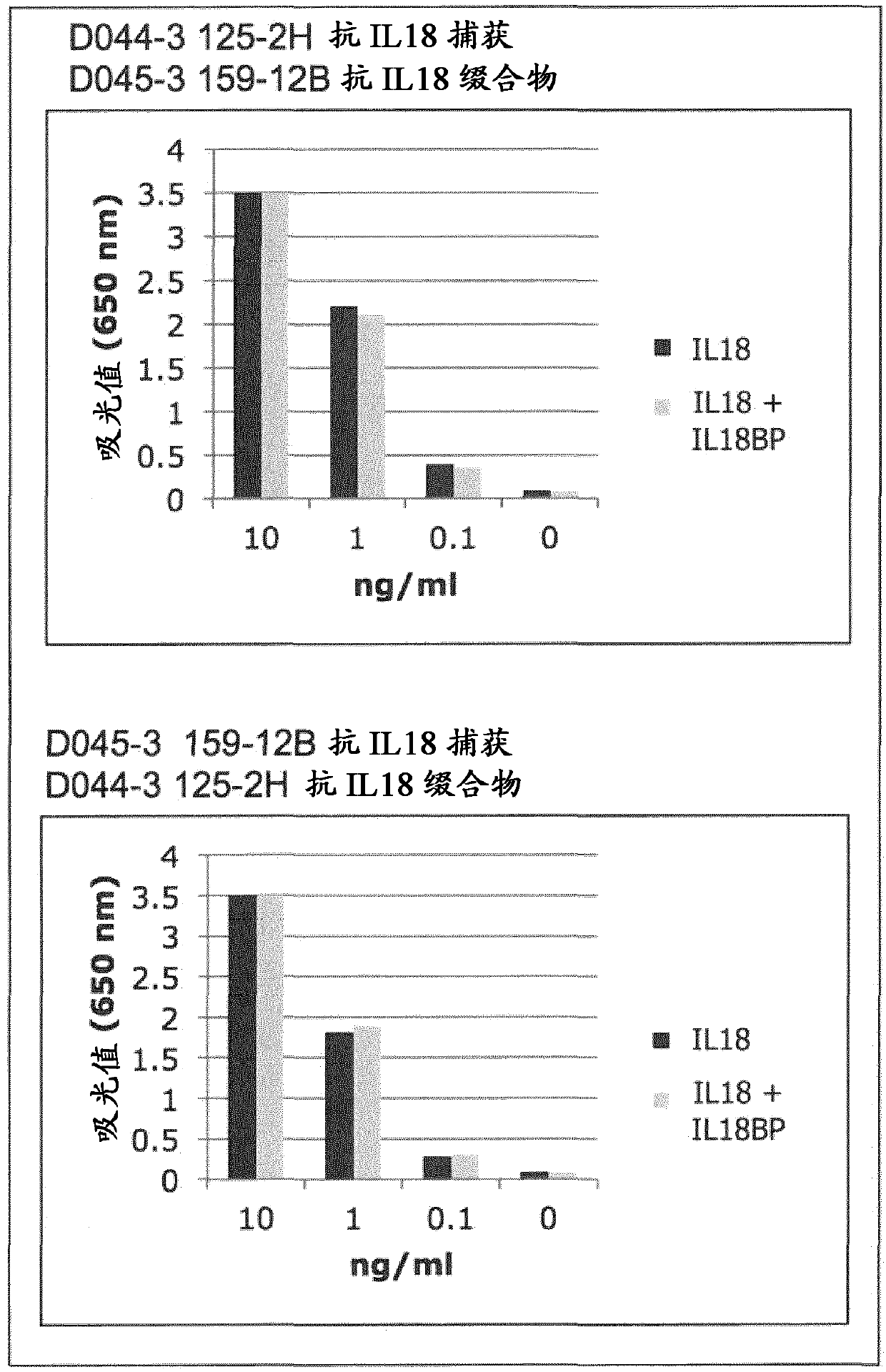
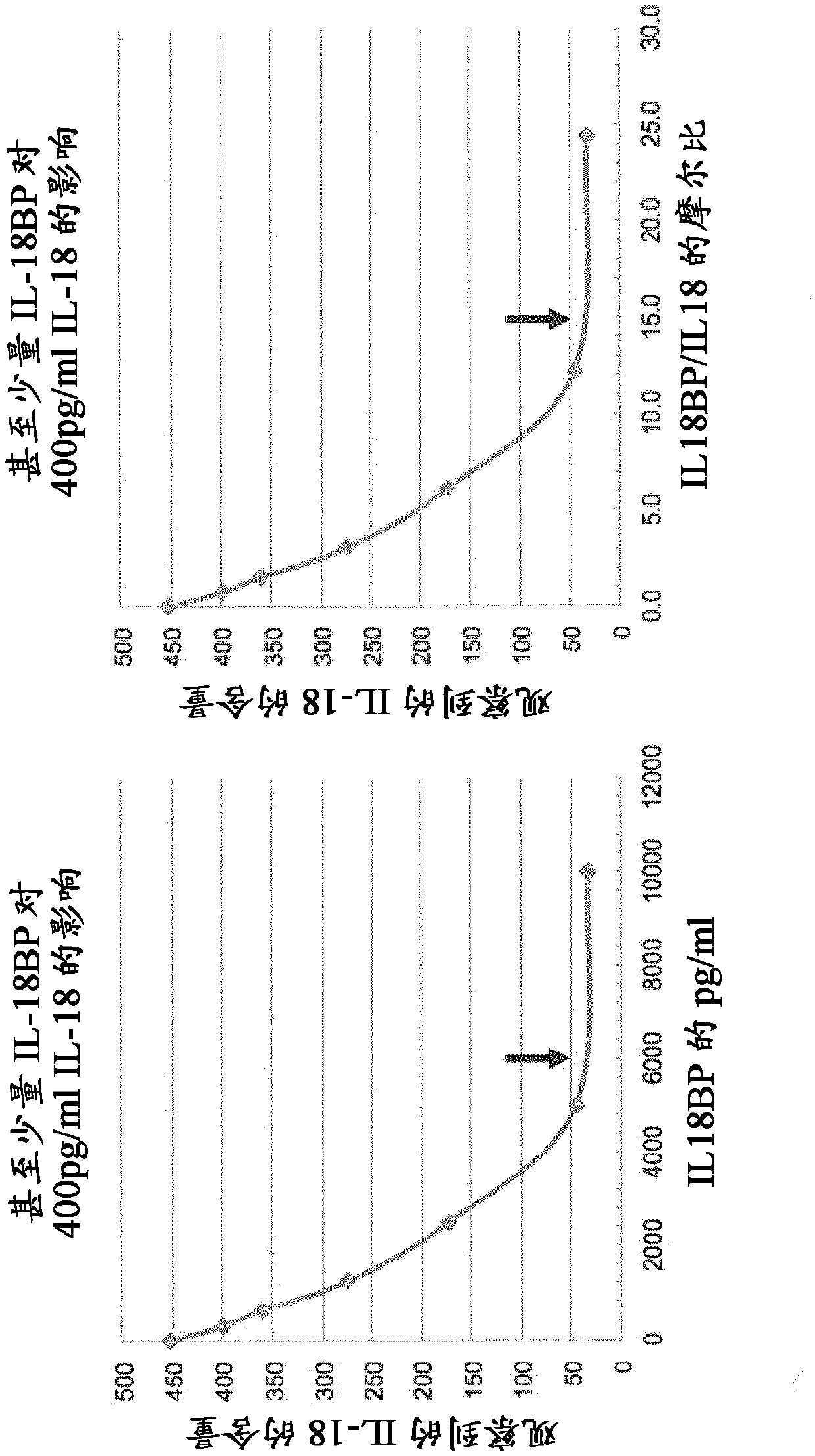
Il-18 Binding Protein (Il-18bp) And Antibodies In Inflammatory Diseases
The present invention provides means and methods for treating Interleukin 18 (IL-18)-associated diseases and disorders, in particular, the present invention discloses antibodies specific for free IL-18 and IL-18 Binding Protein (IL-18BP) for use in such treatments and for the diagnosis of the diseases and disorders.
Owner:AB2 BIO
Eukaryotic coexpression vector of gB gene of avian infectious laryngotracheitis virus and chicken interleukin-18 gene
InactiveCN102220368AAvoid the problem of disproportionate intakeImprove commission efficiencyFermentationVector-based foreign material introductionImmune effectsInfectious laryngotracheitis
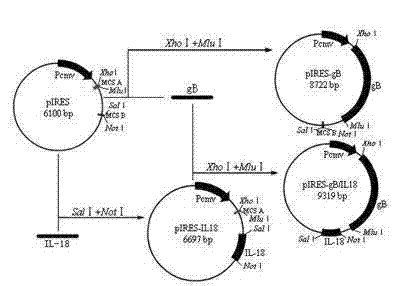
The invention discloses a eukaryotic coexpression vector of a gB gene of avian infectious laryngotracheitis virus and a chicken interleukin-18 gene. The eukaryotic coexpression vector is constructed by respectively or simultaneously inserting the gB gene of avian infectious laryngotracheitis virus (ILTV) and the chicken interleukin 18 (IL-18) gene into a eukaryotic coexpression vector (p1RES), wherein a fragment of the gB gene of the avian infectious laryngotracheitis virus is inserted between the restriction sites XhoI and M1uI of an MCSA (Multiple Cloning Site A) at the downstream of a promoter and a fragment of the chicken interleukin-18 gene is inserted between the restriction sites Sa1I and NotI of an MCSB (Multiple Cloning Site B) to obtain the coexpression plasmid pIRES / gB / IL18. According to the eukaryotic compression vector disclosed by the invention, after a nucleic acid vaccine immune organism is obtained, the expressed gB glycoprotein can stimulate the organism to have an immune protection function on the avian infectious laryngotracheitis virus; the expressed chicken interleukin 18 can give play to the obvious immune adjustment; and the immune effect is enhanced through the synergistic effect of the avian infectious laryngotracheitis virus and the chicken interleukin 18.
Owner:ZHENGZHOU HOUYI PHARMA
IL-18 mutant polypeptide as well as preparation and usage
InactiveCN101177454APeptide/protein ingredientsRecombinant DNA-technologyTemperature controlEscherichia coli
The invention discloses an IL-18 mutant peptide and the preparation method and the usage, which,belongs to the biological technical field. The invention is characterized in that the PCR site-directed mutagenesis technology is adopted to construct the human IL 18 mutant (hIL-18), which is cloned into the temperature-controlling carrier with the PR-PL promoter, and expressed with high efficiency among the Escherichia coli. The expressed hIL-18 mutant D134R is used to cure the impairment caused by IL-18.
Owner:郑骏年
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com