Il-18 Binding Protein (Il-18bp) And Antibodies In Inflammatory Diseases
A 1. IL-18, antibody technology, applied in allergic diseases, antibodies, anti-inflammatory agents, etc., can solve problems such as excessive secretion of constitutive IL-18
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[1117] a. Detection of free IL-18vs. complex IL-18 / IL-18BP
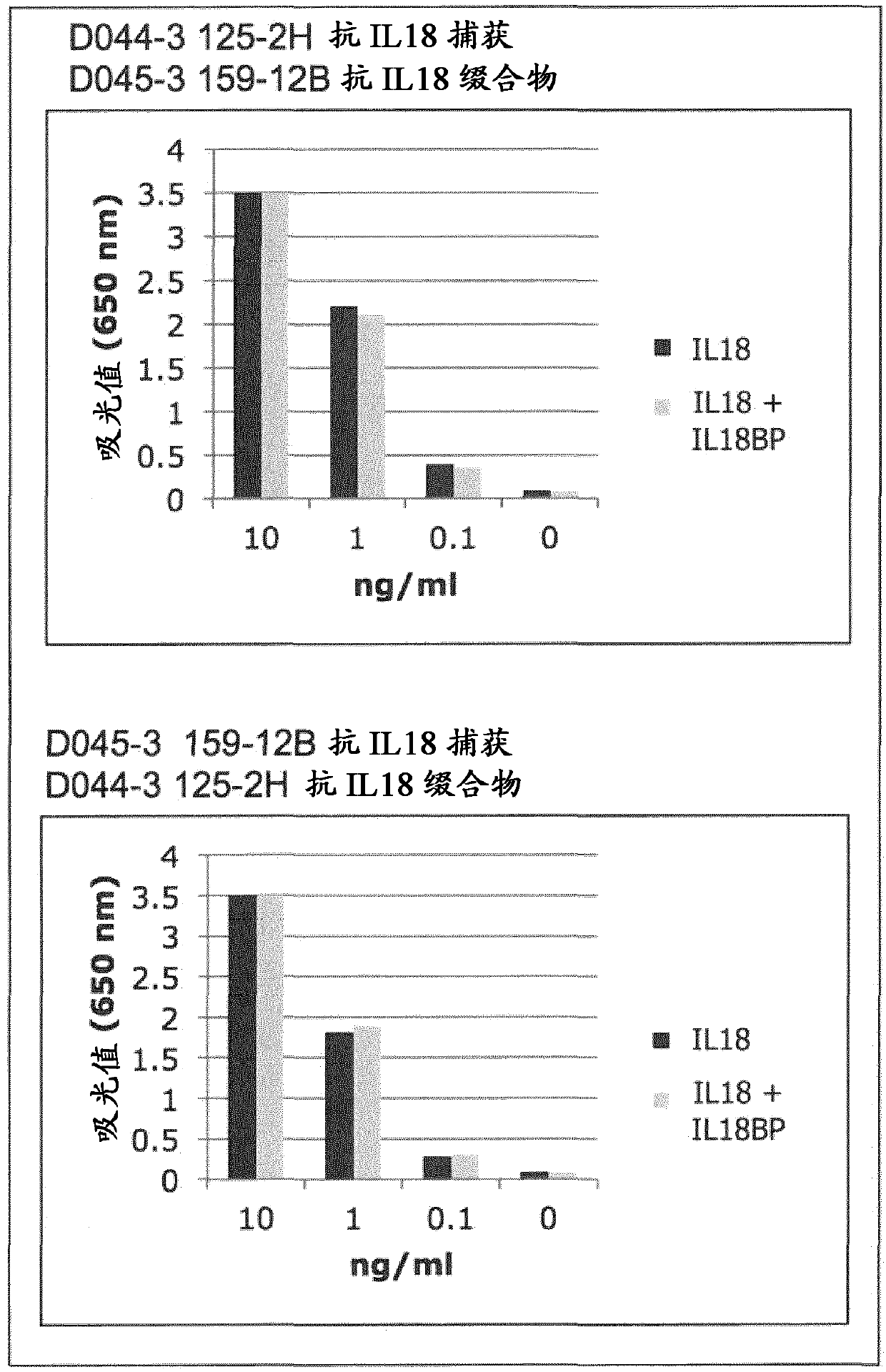
[1118] 1. Common detection of IL-18 in patients
[1119]Quantification of human IL-18 in patients was performed with an ELISA assay detecting total IL-18 (both free form and IL-18BP complex). This ELISA contained commercially available antibodies (see Table 1 below). The most common ELISA assay is performed with anti-IL-18 antibody pairs developed by Taniguchi et al. 1997 and sold by different suppliers, namely mouse mAb 125-2H as primary / capture antibody and rat mAb 159-12B As a secondary / chromogenic antibody.
[1120]
[1121]
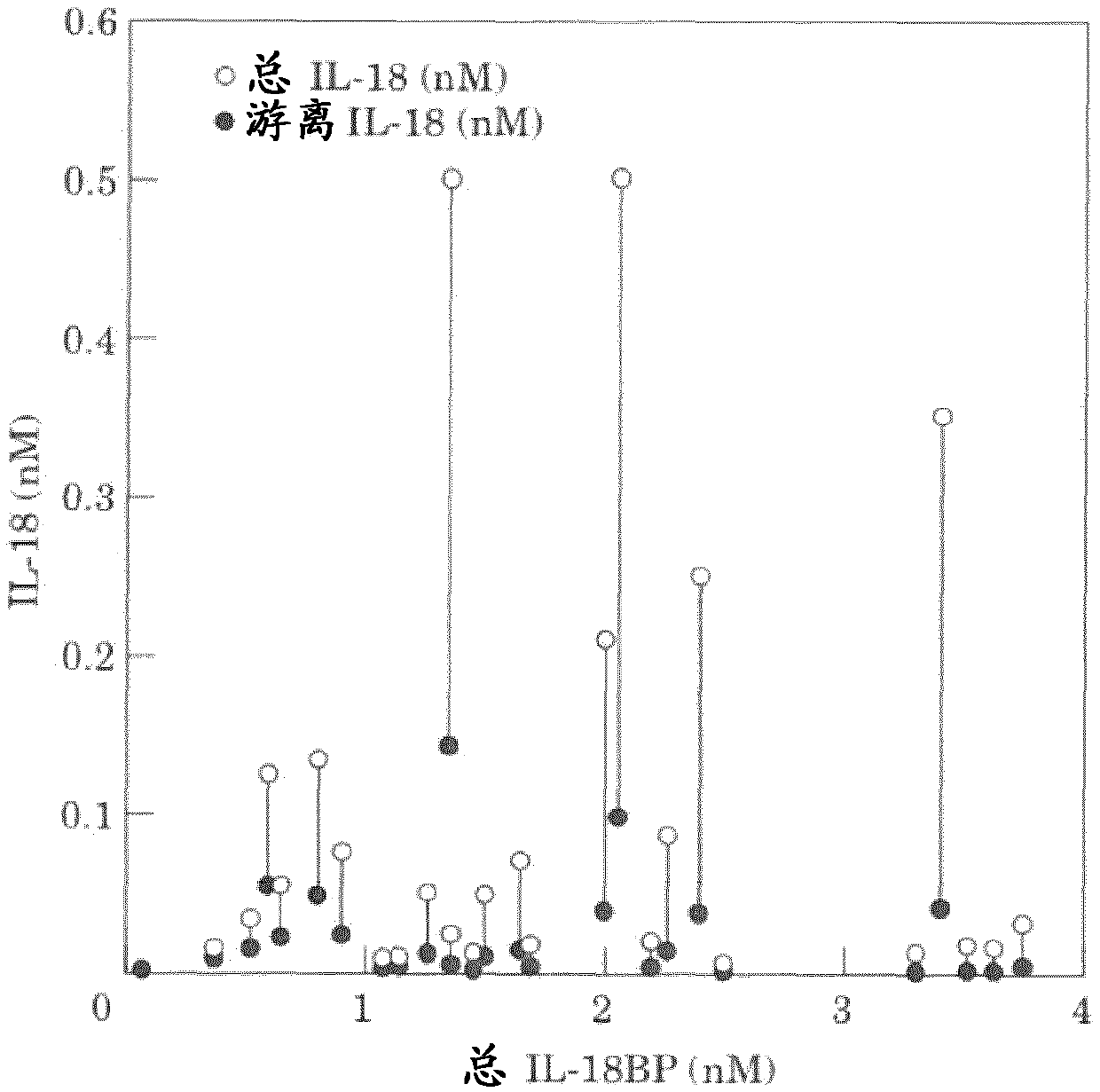
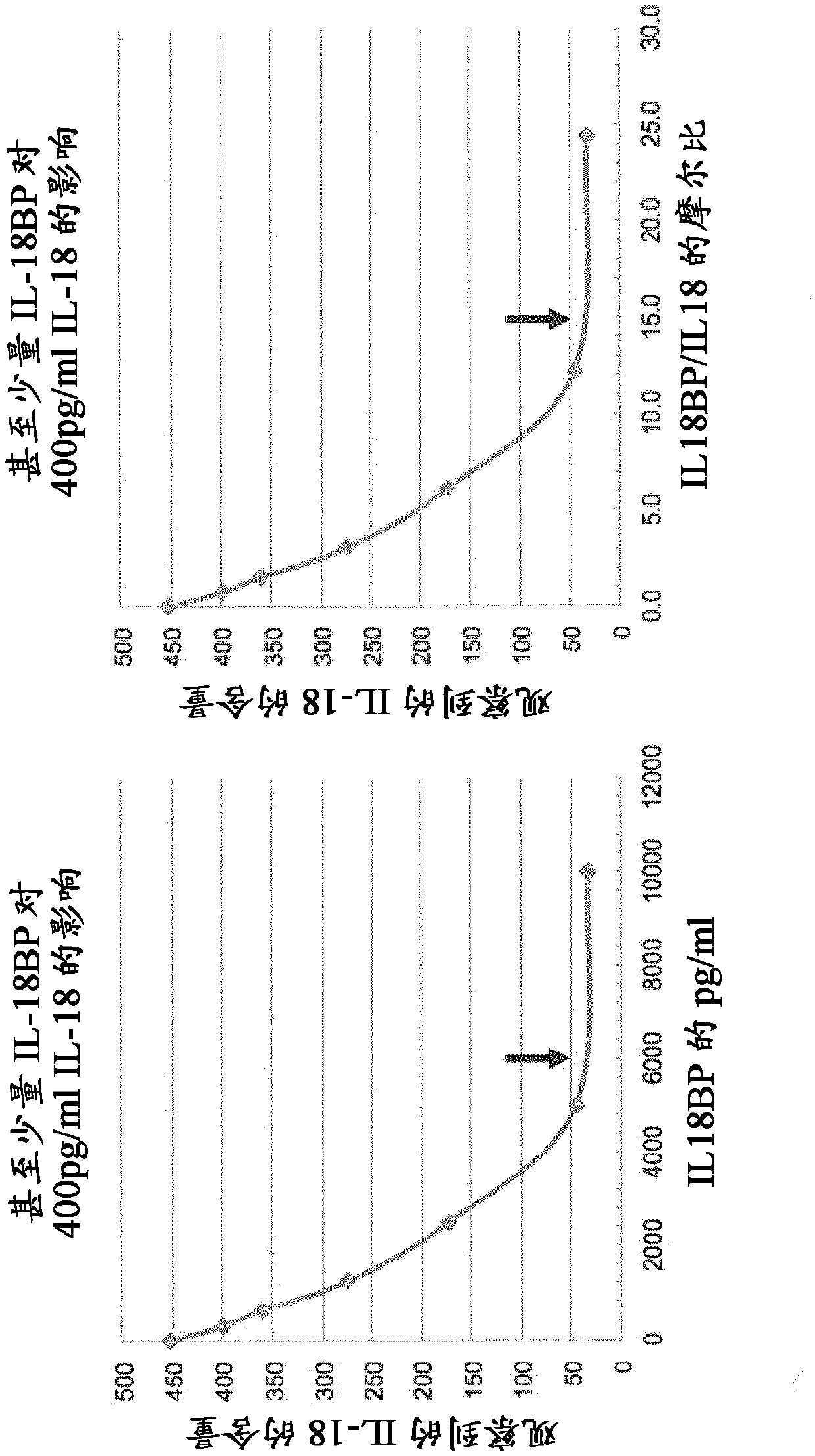
[1122] 2. Estimation of Free IL-18 Levels
[1123] To date, there are no reports of measured levels of free IL-18. Free IL-18 estimates were made by extrapolation using the calculations described by Novick et al. 2004 (see below). The data compare the levels of IL-18 and IL-18BP in humans. In these studies, investigators used commercial monoclonal anti-IL-18 antibody 125-2H an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com