Method for determining radiosensitivity
A sensitive, compound technology, applied in the direction of radiotherapy, X-ray/γ-ray/particle irradiation therapy, treatment, etc., can solve the problem of not having both positive and negative predictive value, lack of technical feasibility, lack of sensitivity and/or specificity sexual issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0148] Example 1: Lymphocyte apoptosis analysis
[0149] The inventors previously developed a rapid and reproducible assay called RILA (Radiation-Induced Lymphocyte Apoptosis), which measures apoptosis of CD4 and CD8 T-lymphocytes after radiation (0.5-8 Gy) by flow cytometry. This measurement is based on the reduction of nuclear DNA fluorescence resulting from specific chromatin changes that accompany apoptosis. In 150 patients, RILA was used as the primary stratification factor in a phase II randomized study of early breast cancer following conventional surgery compared with concurrent or sequential postoperative radiation therapy with letrozole. point) is breast fibrosis (Azria et al., 2010). Patients with RILA >16% were not found to exhibit radiation-induced late effects, indicating a high negative predictive value of this test. All patients with grade 2 or worse subcutaneous fibrosis had <16% RILA, confirming the predictive value of the test. However, among patients wit...
Embodiment 2
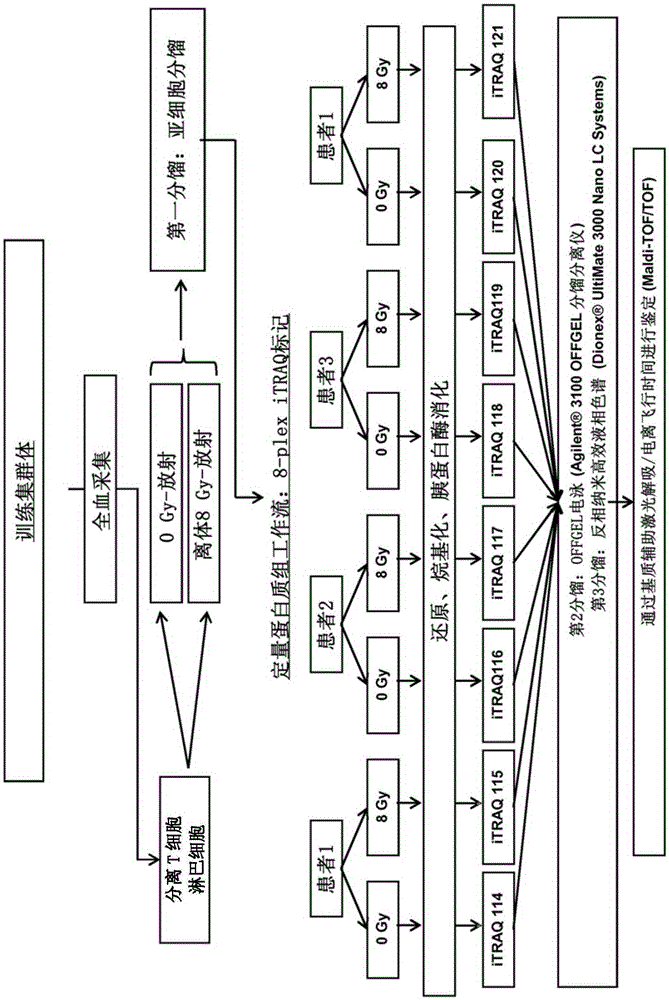
[0150] Example 2: Identification of predictive markers of late-induced cytotoxicity
[0151] The protocol used to identify predictive markers is shown in figure 1 middle. From the aforementioned four patients, a total of 40 ml of blood was collected in heparinized tubes. Use the rosette (rosette) according to the manufacturer's instructions ( StemCell Technology) performed negative selection followed by isolation of T-lymphocytes from whole blood by Ficoll gradients (GE Healthcare). Lymphocytes were then cultured in RPMI medium with 10% FCS at 37°C and 5% CO2 for 24 hours. Subsequently, half of the lymphocytes were irradiated with 8Gy in vitro. Then, irradiated and non-irradiated lymphocytes were cultured again for 48 hours at 37°C and 5% CO2. After this incubation time, lymphocytes from each patient were then submitted to subcellular fractionation (fraction) ( The Subcellular Proteome Extraction Kit (cat. 539790, Merckmillipore) allows the separation of cytoplasmic, m...
Embodiment 3
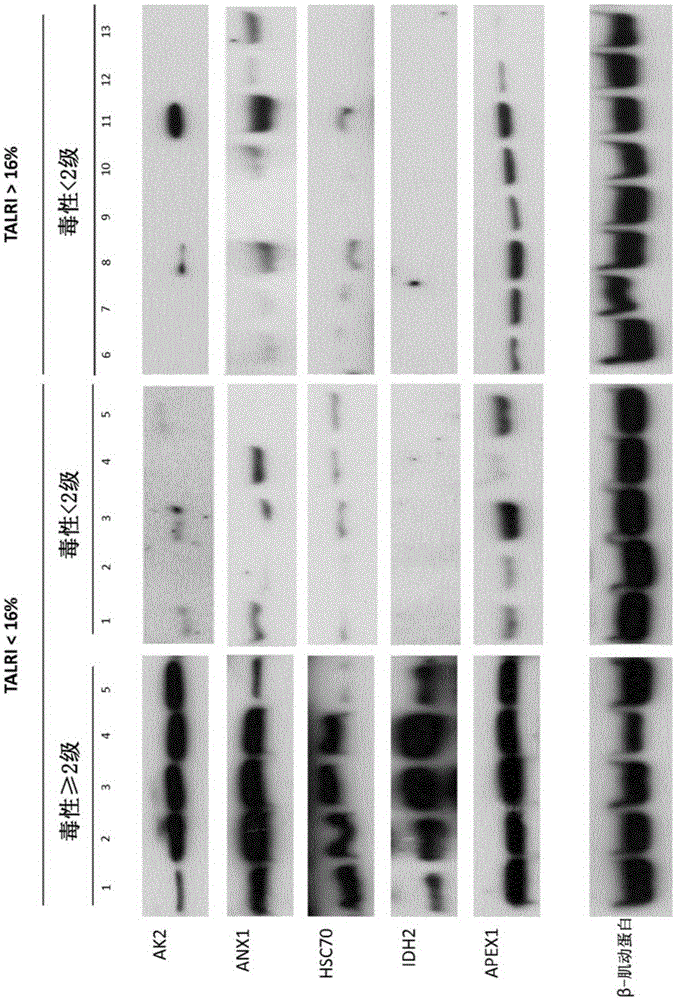
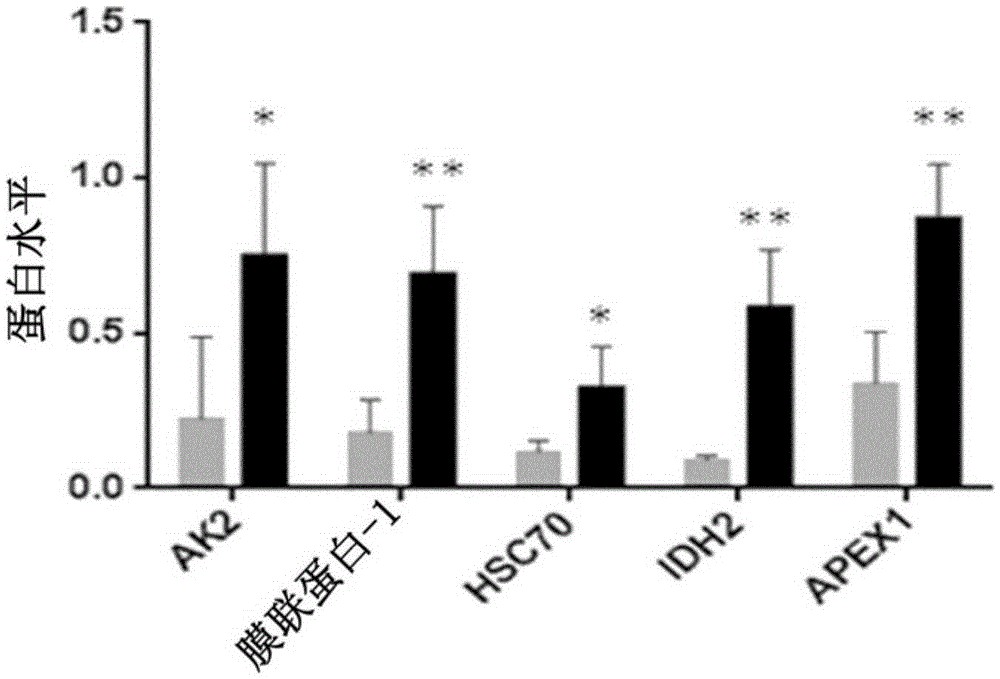
[0156] Example 3: Confirmation of differential expression of biomarkers in a larger number of patients following radiation therapy
[0157] These five proteins were validated by western blot analysis in an additional population of 18 patients, of which 5 patients had developed > grade 2 breast fibrosis and 13 patients had only mild or no toxicity. All these 10 patients exhibited low RILA values. Blood samples were collected and processed until post-irradiation incubation as described in previous examples. Then, lymphocytes were lysed in RIPA buffer. Then, the protein was quantified again, and each 10 μg was placed in a 12% polyacrylamide gel for Western blotting. After migration, transfer to PVDF membrane at 300mA for 1 hour at 4°C, then saturate the membrane for 2 hours in PBS-Tween 0.05%-milk 5%, the antibody against the protein of interest in the same saturation buffer in Incubate overnight at 4°C with agitation. After five consecutive 5 min washes in PBS-Tween 0.05% bu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com