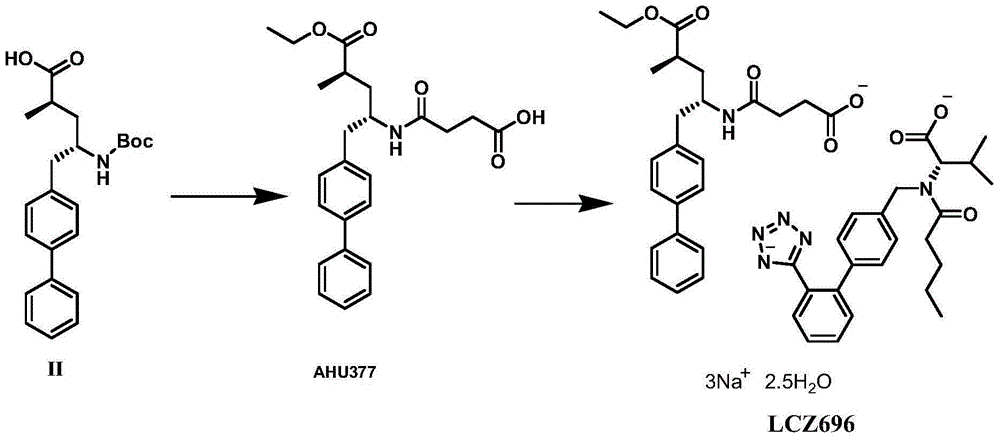
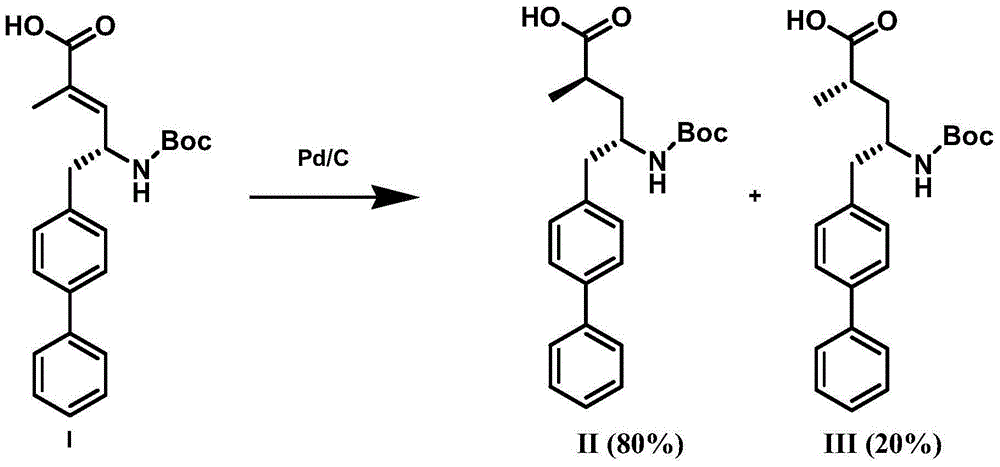
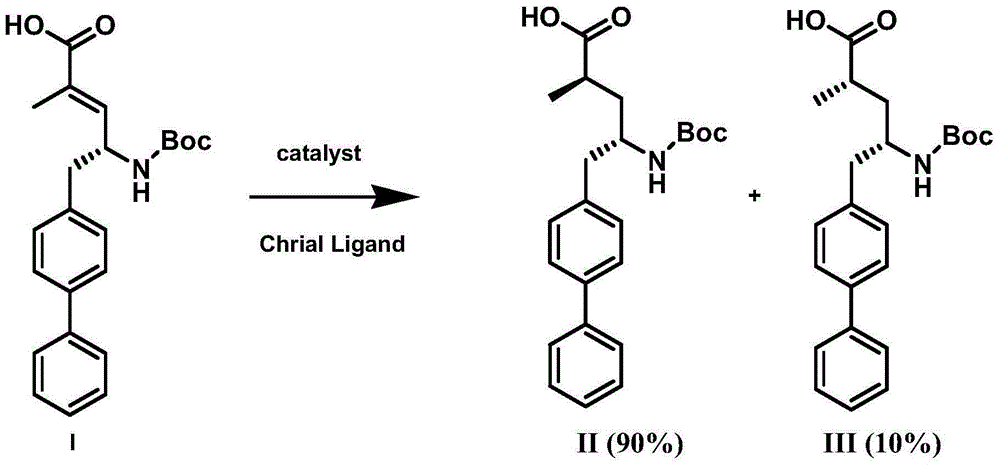
Preparation method of NEP (neutral endopeptidase) inhibitor intermediate
An intermediate and inhibitor technology, applied in the field of industrial production and preparation, can solve the problems of expensive reagents, difficult ligand synthesis, and unfavorable industrial scale-up production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Add (R,E)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)amino)-2-methanol to the autoclave in sequence Base-2-pentenoic acid 381g, ethanol 3.5kg, bis(dicyclopentadiene)rhodium tetrafluoroborate 99mg and phosphine ligand CK-01221mg, replace hydrogen, pressure 15kg, temperature 40°C, stir until LC control reaction Completely, filtered, and the filtrate was spin-dried to obtain the crude product, and the two isomers II:III=82:18 were analyzed by chiral analysis. The crude product was washed with ethyl acetate and petroleum ether, and then recrystallized with ethanol to obtain the product (2R,4S)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxy Carbonyl)amino)-2-methylpentanoic acid 307g.
[0039]
Embodiment 2
[0040] Example 2: Add (R,E)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)amino)-2-methanol to the autoclave in sequence Base-2-pentenoic acid 381g, ethanol 3.5kg, bis(dicyclopentadiene) rhodium tetrafluoroborate 99mg and phosphine ligand CK-02230mg, replace hydrogen, pressure 15kg, temperature 40°C, stir until LC control reaction complete, filtered, and the filtrate was spin-dried to obtain the crude product, and the two isomers II:III=85:15 were analyzed by chiral analysis. The crude product was slurried with ethyl acetate and petroleum ether, then recrystallized with ethanol, and the crude product was slurried with ethyl acetate and petroleum ether to obtain the product (2R,4S)-5-([1,1'-biphenyl]-4- Base)-4-((tert-butoxycarbonyl)amino)-2-methylpentanoic acid 280g.
[0041]
Embodiment 3
[0042] Example 3: Add (R,E)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)amino)-2-methanol to the autoclave in sequence Base-2-pentenoic acid 381g, ethanol 3.5kg, bis(dicyclopentadiene)rhodium tetrafluoroborate 99mg and phosphine ligand CK-04279mg, replace hydrogen, pressure 15kg, temperature 40°C, stir until LC control reaction complete, filtered, and the filtrate was spin-dried to obtain the crude product, and the two isomers II:III=90:10 were analyzed by chiral analysis. The crude product was slurried with ethyl acetate and petroleum ether, and then recrystallized with ethanol, and the crude product was slurried with ethyl acetate and petroleum ether to obtain the product (2R,4S)-5-([1,1'-biphenyl]-4- Base)-4-((tert-butoxycarbonyl)amino)-2-methylpentanoic acid 340g.
[0043]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com