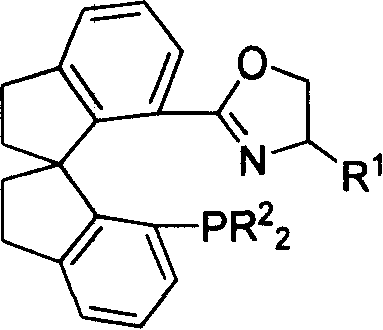
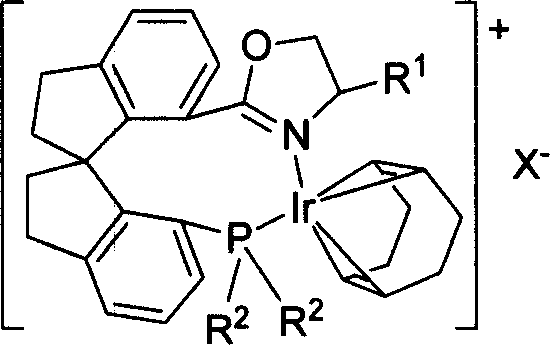
Phosphorus-oxazoline ligand with spiro backbone and its uses in asymmetrical catalytic hydrogenation
A technology of oxazoline and ligand is applied in the synthesis field of novel chiral spirocyclic phosphine-oxazoline ligand and its ionic iridium complex, and achieves the effect of high stereoselectivity and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
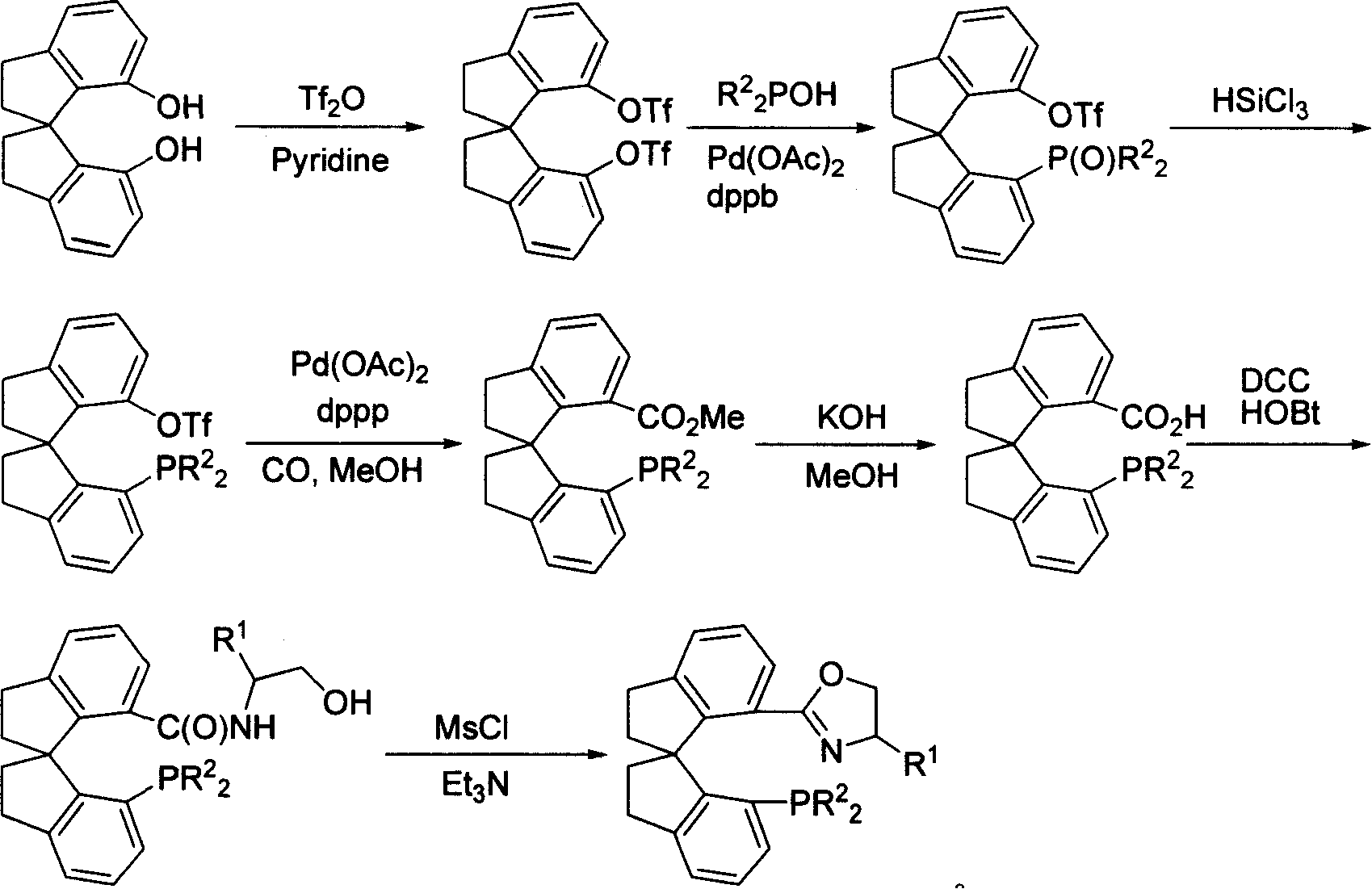
[0034] Example 1: Preparation of (S)-7,7'-bis(trifluoromethanesulfonyloxy)-1,1'-spirodihydroindane
[0035]
[0036] Add (S)-1,1'-spirodihydroindane-7,7'-diphenol (5.0g, 19.8mmol), pyridine (7.0mL, 86.7mmol) and 100mL dichloromethane into a 250mL reaction flask, ice The salt bath was cooled to below 0°C, and trifluoromethanesulfonic anhydride (8.2 mL, 43.7 mmol) was added dropwise from a constant pressure dropping funnel under temperature control. After the addition, the mixture was naturally warmed to room temperature and stirred overnight. Rotary evaporation precipitation, the residue was dissolved in 80mL ethyl acetate, transferred to a separatory funnel, and 5% HCl solution, saturated saline, saturated NaHCO 3 solution, washed with saturated brine successively, anhydrous Na 2 SO 4 dry. After filtering and removing the solvent, an appropriate amount of dichloromethane was dissolved, passed through a short column of silica gel, and rinsed with dichloromethane. The so...
Embodiment 2
[0037] Example 2: Preparation of (S)-7-diphenylphosphono-7'-trifluoromethanesulfonyloxy-1,1'-spirodihydroindene
[0038]
[0039] Add (S)-7,7'-bis(trifluoromethanesulfonyloxy)-1,1'-spirodihydroindane (4.0 g, 7.75 mmol), diphenylphosphine oxide (3.13 g, 15.5 mmol), palladium acetate (87 mg, 0.39 mmol), 1,4-bis(diphenylphosphino)ylbutane (dppb, 166 mg, 0.39 mmol), and 25 mL of degassed DMSO. Electromagnetic stirring was used to make it fully mixed. After adding N,N-diisopropylethylamine (4.1 g, 32 mmol), the mixture was heated at 100° C. in an oil bath and reacted for 6 hours. Cool to room temperature, dilute with ethyl acetate, separate, 5% HCl solution, saturated saline, saturated NaHCO 3 solution, washed with saturated brine successively, anhydrous Na 2 SO 4 dry. After filtering and removing the solvent, use silica gel column chromatography (eluent: petroleum ether / EtOAc=3:1) to obtain (S)-7-diphenylphosphono-7'-trifluoromethanesulfonyloxy - 4.0 g of 1,1'-spiroindane,...
Embodiment 3
[0040] Example 3: Preparation of (S)-7-bis(p-methoxyphenyl)phosphono-7'-trifluoromethanesulfonyloxy-1,1'-spiroindene
[0041] Prepared with (S)-7,7'-bis(trifluoromethanesulfonyloxy)-1,1'-spirodihydroindane and bis-p-methoxyphenylphosphonous acid, the method is the same as in Example 2 same. A white solid was obtained, yield: 90%. Mp 150-152°C; [α] 8 D -216(c 0.5, CH 2 Cl 2 ); 1 H NMR (300MHz, CDCl 3 )δ2.20-2.32 (m, 3H, CH 2 ), 3.04-3.18 (m, 3H, CH 2 ), 3.20-3.40 (m, 2H, CH 2 ), 3.78(s, 3H, OCH 3 ), 3.85(s, 3H, OCH 3 ), 6.24(d, 2H, J=8.1Hz, Ar-H), 6.80-6.85(m, 4H, Ar-H), 6.86-7.00(m, 2H, Ar-H), 7.16-7.21(m, 4H, Ar-H), 7.21-7.30 (m, 2H, Ar-H), 7.32 (d, 1H, J=7.2Hz, Ar-H); 31 PNMR (121MHz, CDCl 3 )δ31.45(s); 13 C NMR (75MHz, CDCl 3 )δ29.7, 30.1, 30.8, 38.7, 38.9, 54.2, 60.8, 112.4, 112.6, 116.3, 118.8, 121.1, 122.6, 124.9, 125.1, 125.9, 126.4, 126.9, 127.2, 127.4, 127.8, 132.9 , 132.5, 139.7, 143.8, 144.8, 145.0, 148.6, 151.7, 160.7, 160.9; MS (EI) m / z 628 (M + ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com