Preparation method of mono-chiral metallic organic frame material with function of splitting chiral amine
A metal-organic framework, chiral amine technology, applied in the field of preparation of monochiral metal-organic frameworks (MOFs), to achieve the effects of high efficiency, stable internal structure and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
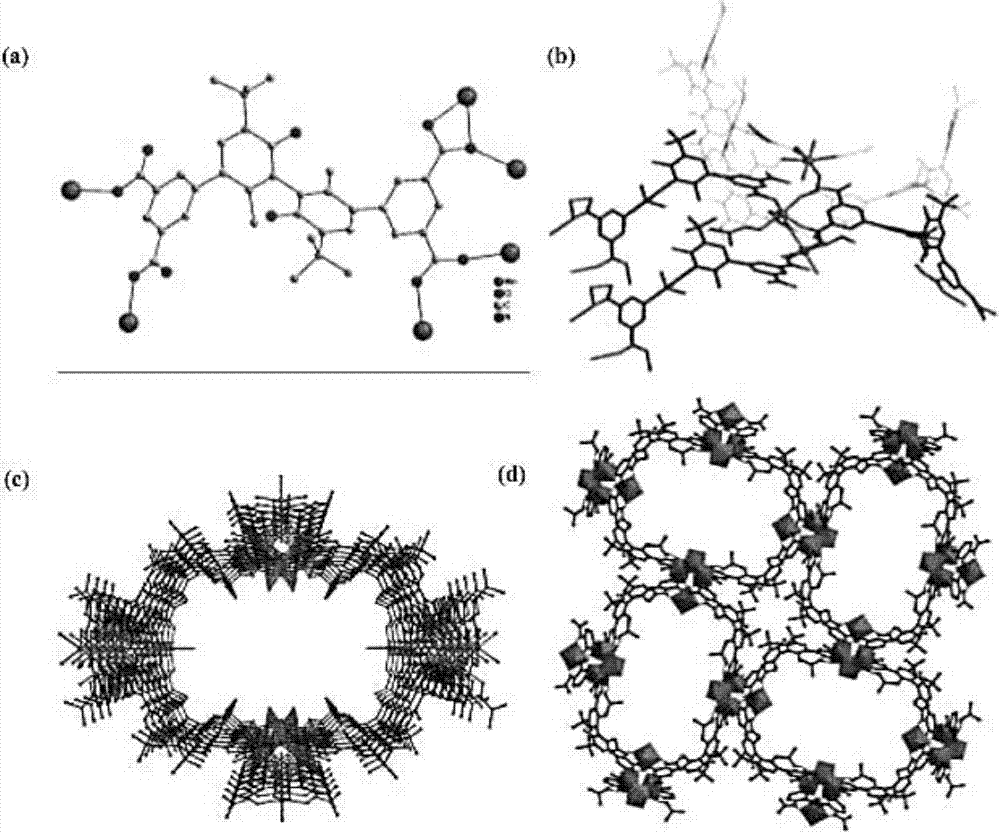
Image
Examples
Embodiment 1
[0041] Step 1, (S)-3,3'-di-tert-butyl-5,5'-dibromo-6,6'-dimethyl-2,2'-dihydroxy-1,1'-biphenyl [Eur.J.Org.Chem.2010, 3027] (1.0g, 2mmol), 5-pinacol ester methyl isophthalate [Cryst.Growth Des., 2010, 2775] (1.7g, 5.6mmol) , potassium phosphate trihydrate (1.9g, 9mmol), [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (80mg, 0.1mmol) was placed in a reaction flask, and added under nitrogen protection Dioxane / water = 9:1 (60mL) mixed solvent, reflux for 10 hours and then cool to room temperature, extract the reaction solution several times with ethyl acetate, combine the organic phases and wash with saturated sodium chloride for several times After drying with anhydrous sodium sulfate, it was filtered with a Buchner funnel and spin-dried, and the crude product was separated and purified by column chromatography (silica gel column, the eluent was petroleum ether:ethyl acetate=10:1) to obtain the target product (S )-3,3'-di-tert-butyl-5,5'-bis(3,5-dimethoxycarbonylphenyl...
Embodiment 2
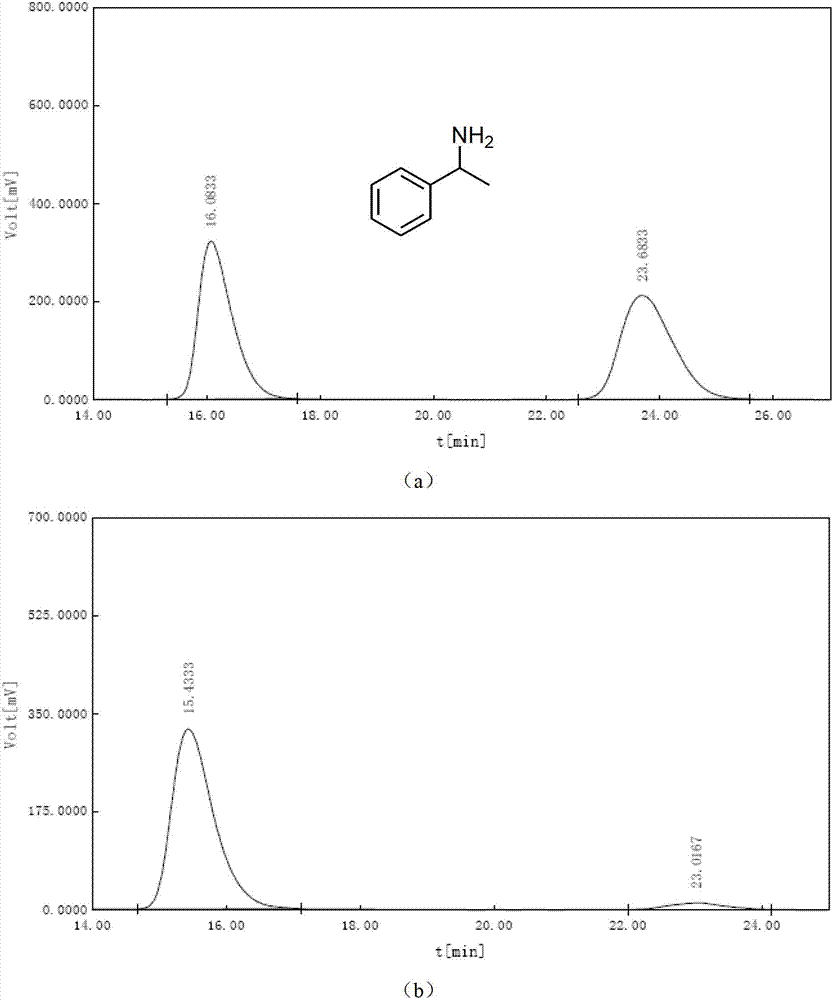
[0052]After solvent exchange, 30 mg of the monochiral metal-organic framework material with chiral separation function obtained in Step 3 of Example 1 was placed under vacuum at 80°C for 2 hours, and then dissolved in 5 mL of exosome at -10°C. Racemic 1-(4-fluorophenyl)ethylamine soaked in methanol for one day, filtered through a Buchner funnel, washed several times with methanol, then soaked in tetrahydrofuran for one day, filtered through a Buchner funnel, and the filtrate was derivatized with benzoyl chloride Using a Chiralcel OD-H liquid-phase chiral separation column, at 25°C, n-hexane / isopropanol=90 / 10, and a flow rate of 0.6mL / min, the ee value of chiral amine separation can reach 98.7%, as shown in image 3 shown. image 3 The analysis results in are as follows:
[0053]
Embodiment 3
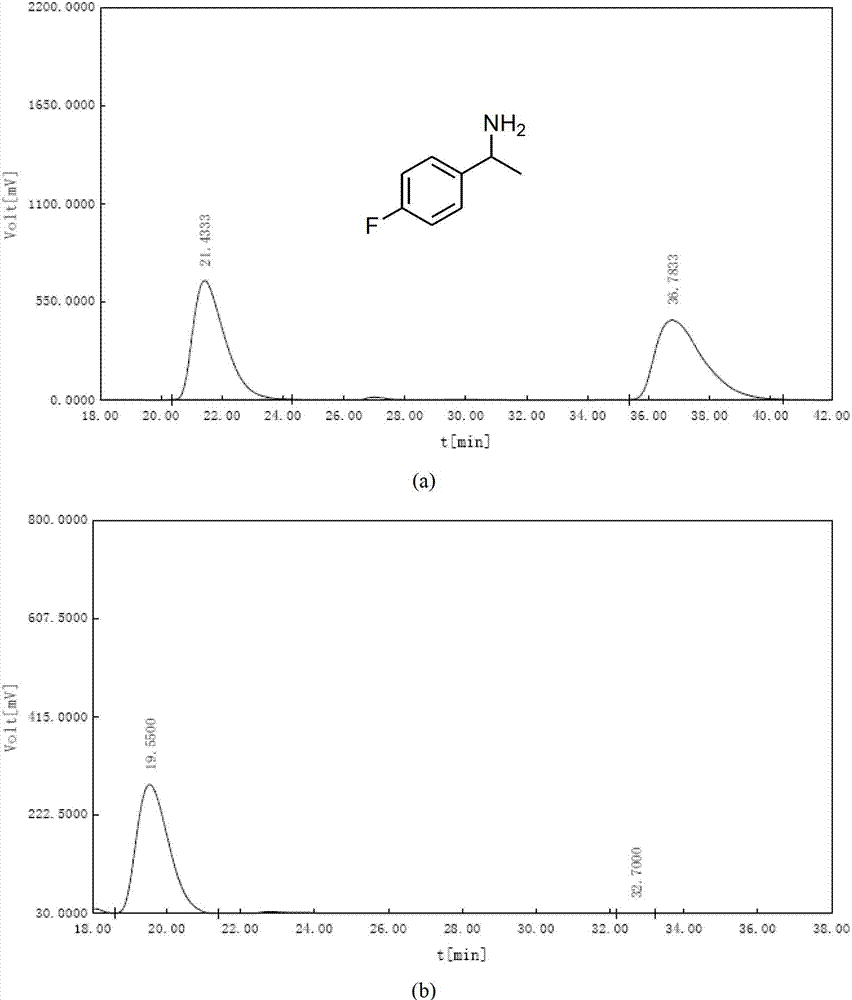
[0055] After solvent exchange, 30 mg of the monochiral metal-organic framework material with chiral separation function obtained in Step 3 of Example 1 was placed under vacuum at 80°C for 2 hours, and then dissolved in 5 mL of exosome at -10°C. Soak racemic 1-(4-bromophenyl)ethylamine in methanol for one day, filter with Buchner funnel, wash with methanol several times, then soak in tetrahydrofuran for one day, filter with Buchner funnel, and derivatize the filtrate with benzoyl chloride Using a Chiralcel OD-H liquid-phase chiral separation column, at 25°C, n-hexane / isopropanol=90 / 10, and a flow rate of 1.0mL / min, the measured chiral amine separation ee value reaches 91.5%, such as Figure 4 shown. Figure 4 The analysis results in are as follows:
[0056]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com