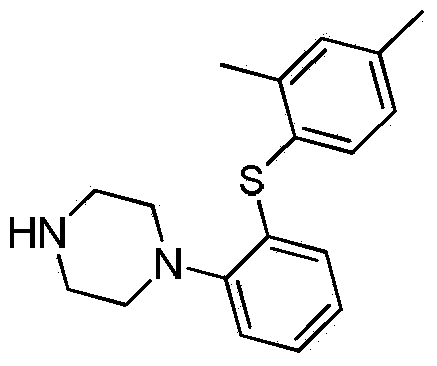
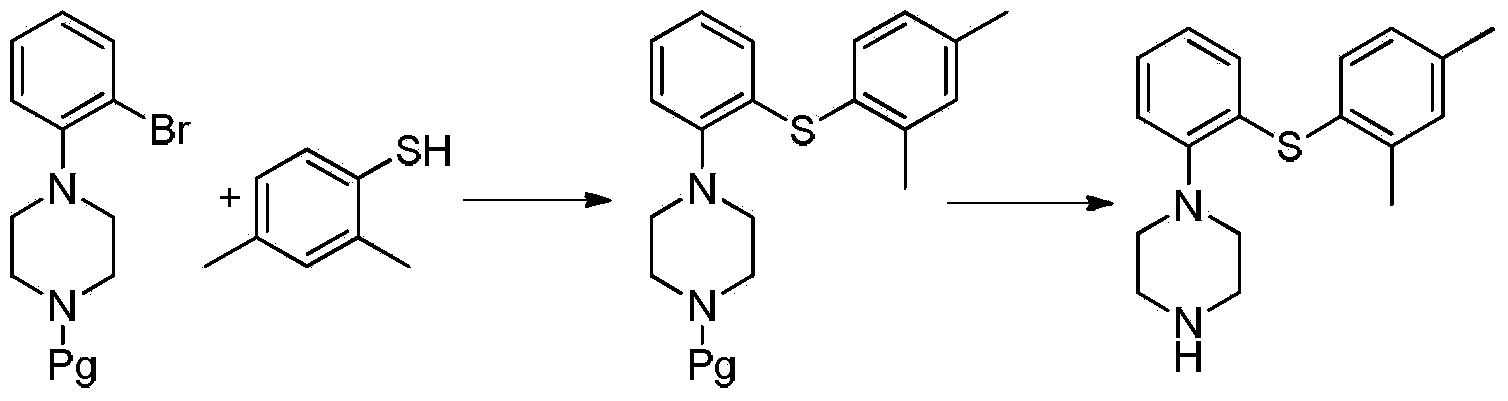
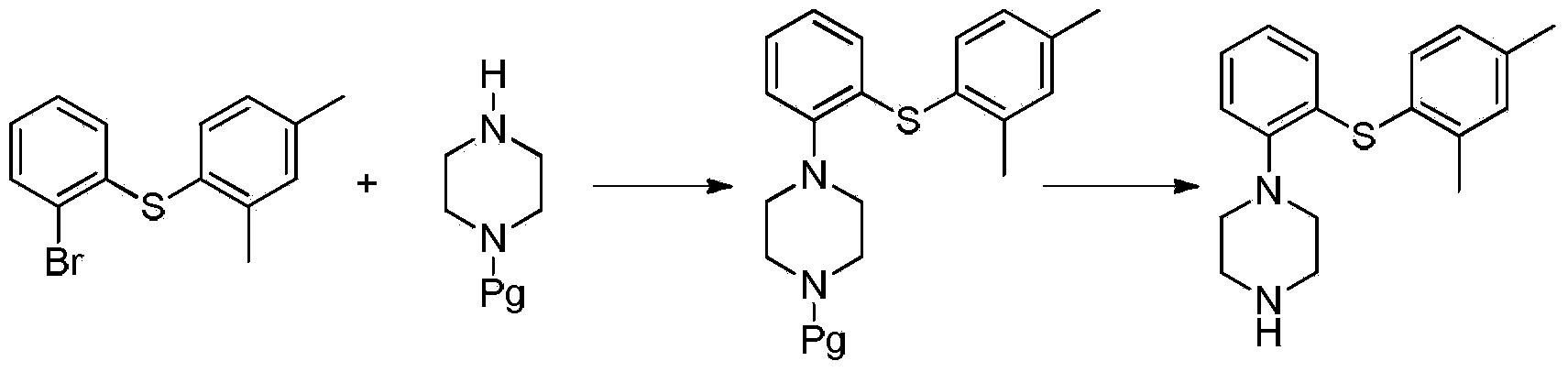
Preparation method of Vortioxetine
A technology of vortioxetine and compound, applied in the field of preparation of vortioxetine, can solve the problem of not fundamentally solving the problem of competitive side reaction of dihalogen, and achieve the problem of solving the problem of competitive side reaction, easy availability of raw materials, and by-products. less effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Under nitrogen protection, 13.8g (0.1mol) compound 2,4-dimethylthiophenol, 28.3g (0.1mol) 2-bromoiodobenzene, 53.0g (0.25mol) were successively put into a 500mL reactor Potassium phosphate and 1.9g (0.01mol) cuprous iodide, 1.15g (0.01mol) L-proline, add 300mL dimethylformamide, stir and heat up to 70-80°C, react for 5h, after the reaction is completed, Add 9.46g (0.11mol) piperazine to the reaction solution, continue the heating reaction for 5-8 hours, steam off part of the solvent after the reaction is completed, add water to the residue, extract three times with dichloromethane, each 300ml, combine the organic layers, Dry, evaporate dichloromethane, recrystallize by adding 200mL acetonitrile to the residue, obtain white solid 28.8g, yield 96.7%, purity 99.2%, maximum simplex 0.05% [HPLC method: chromatographic column Luna Phenyl-Hexyl column (4.6mmx150mm , 3um); mobile phase acetonitrile: 0.05% trifluoroacetic acid solution (60:40); detection wavelength 226Din; colum...
Embodiment 2
[0041]Under nitrogen protection, 13.8g (0.1mol) compound of formula (4) 2,4-dimethylthiophenol, 28.3g (0.1mol) 2-bromoiodobenzene, 63.6g (0.3mol) were successively put into a 500mL reactor Potassium phosphate and 3.8g (0.02mol) cuprous iodide, 2.3g (0.02mol) L-proline, add 300mL dimethylformamide, stir and raise the temperature to 70-80°C, react for 5h, after the reaction is completed, Add 1.03g (0.12mol) piperazine to the reaction solution, continue the heating reaction for 5-8 hours, evaporate part of the solvent after the reaction is completed, add water to the residue, extract three times with dichloromethane, each 300ml, combine the organic layers, After drying, the dichloromethane was distilled off, and the residue was recrystallized by adding 200 mL of acetonitrile to obtain 28.1 g of a white solid, with a yield of 93.7%, a purity of 99.1%, and a maximum of 0.08% impurity.
Embodiment 3
[0043] In the 500mL reactor, drop into 13.8g (0.1mol) formula (4) compound 2,4-dimethylthiophenol, 28.3g (0.1mol) 2-bromoiodobenzene, 53.0g (0.25mol) potassium phosphate and 1.9 g (0.01mol) cuprous iodide, 1.14g (0.01mol) (S,S)-cyclohexanediamine, add 300mL dimethylformamide, heat up to 70-80°C under stirring, react for 5h, after the reaction is completed , add 9.46g (0.11mol) piperazine to the reaction solution, continue to heat the reaction for 5-8 hours, evaporate part of the solvent after the reaction is completed, add water to the residue, extract three times with dichloromethane, 300ml each time, and combine the organic layers , dried, dichloromethane was distilled off, and the residue was recrystallized by adding 200 mL of acetonitrile to obtain 27.9 g of a white solid with a yield of 93.6%, a purity of 98.8%, and a maximum purity of 0.15%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com