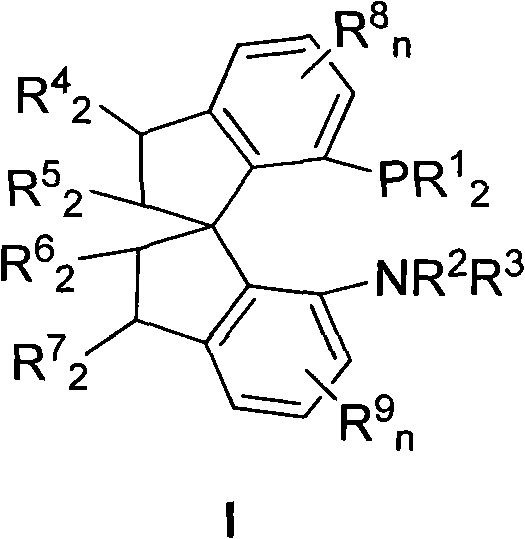
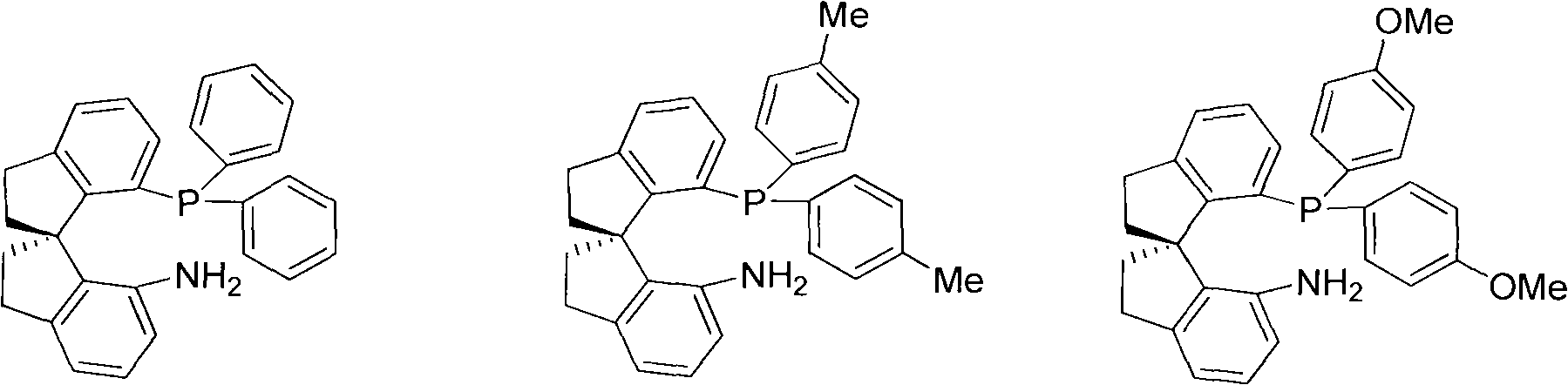
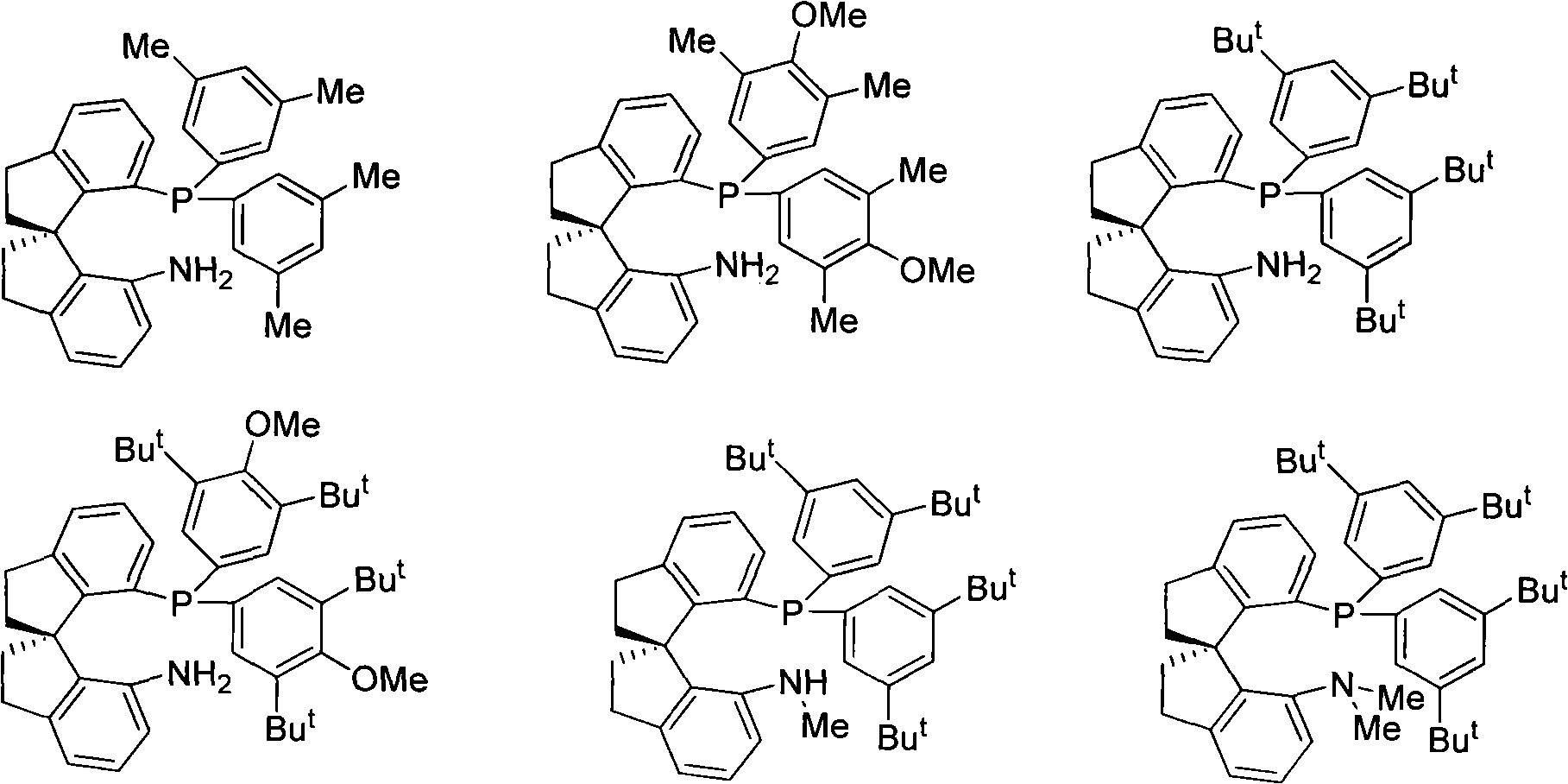
Chiral spiro aminophosphine ligand compound and synthesis method as well as application thereof
A spiro-cyclic aminophosphine and spiro-amino-based technology, which is applied in the field of synthesis of chiral phosphine compounds, can solve problems such as unsatisfactory results and limited types, and achieve the effect of excellent reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of (R)-7-diphenylphosphino-7'-amino-1,1'-spiroindane (Ia-1)
[0035]
[0036] Under the protection of nitrogen, add 48mL of anhydrous chloroform into a 100mL two-neck bottle containing 0.50g (R)-II-1, stir well, and then add 1.6mL of concentrated sulfuric acid at one time. After heating the oil bath to 40-45°C, add 0.33g of sodium azide in batches. After adding sodium azide, reflux the oil bath and stir for four hours; remove the oil bath, cool to 0°C with ice water, add 100 mL of water to dilute the reaction, and adjust the pH value to slightly alkaline with sodium hydroxide solution; chloroform extraction, anhydrous Magnesium sulfate was dried; the magnesium sulfate was removed by filtration, and after drying, precipitation and silica gel column chromatography (ethyl acetate / petroleum ether=1 / 10) gave 0.46 g of white solid, yield 98%.
[0037] M.p.116-118°C; [α] D 20 +212(c 0.39, CH 2 Cl 2 ); 1 H NMR (300MHz, CDCl 3 )δ2.12-2.17 (m, 1H, CH 2 ), 2.2...
Embodiment 2
[0039] Synthesis of (R)-7-bis(p-methylphenyl)phosphino-7'-amino-1,1'-spirodihydroindane (Ia-2):
[0040]
[0041] Refer to Example 1 for specific operation, white solid, yield 80%.
[0042] M.p.108-110°C; [α] D 20 +184(c0.52, CH 2 Cl 2 ); 1 H NMR (300MHz, CDCl 3 )δ2.11-2.36 (m, 4H, CH 2 ), 2.27 (d, 6H, CH 3 ), 2.77(s, 2H, NH 2 ), 2.93-3.02 (m, 4H, CH 2 ), 6.06(d, 1H, Ar-H), 6.68(d, 1H, Ar-H), 6.87-7.23(m, 12H, Ar-H); 31 P NMR (121MHz, CDCl 3 )δ-23.80(s); 13 C NMR (75MHz, CDCl 3 )δ21.2, 21.3, 30.9, 31.2, 36.3, 39.3, 39.3, 61.5, 61.5, 114.2, 115.0, 125.8, 127.4, 128.1, 128.7, 128.9, 128.9, 133.1, 133.3, 134.0, 1364.2, 134.2, , 138.3, 142.5, 142.6, 144.3, 144.4, 144.7, 144.7, 152.8, 153.1, 162.3; HRMS (ESI) calcd for [C 31 h 30 NP+H] + : 448.2189; Found 448.2195.
Embodiment 3
[0044] Synthesis of (R)-7-bis(p-methoxyphenyl)phosphino-7'-amino-1,1'-spirodihydroindene (Ia-3)
[0045]
[0046] Refer to Example 1 for specific operation, white solid, yield 80%.
[0047] M.p.142-144°C; [α] D 20 +178(c 0.40, CH 2 Cl 2 ); 1 H NMR (300MHz, CDCl 3 )δ2.11-2.16 (m, 1H, CH 2 ), 2.21-2.36 (m, 3H, CH 2 ), 2.77(s, 2H, NH 2), 2.93-3.02 (m, 4H, CH 2 ), 3.76 (d, 6H, OCH 3 ), 6.07(d, 1H, Ar-H), 6.69-7.24(m, 12H, Ar-H); 31 P NMR (121MHz, CDCl 3 )δ-25.09(s); 13 C NMR (75MHz, CDCl 3 )δ30.9, 31.2, 36.3, 39.0, 39.1, 55.1, 55.2, 61.5, 61.5, 113.3, 113.7, 113.8, 113.9, 114.1, 115.0, 125.7, 127.4, 128.0, 130.0, 130.1, 133.8, 134.8, 134.5, , 135.6, 142.6, 144.3, 144.4, 144.7, 144.7, 152.5, 152.8, 159.6, 160.1; HRMS (ESI) calcd for [C 31 h 30 NO 2 P+H] + : 480.2087; Found 480.2095.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com