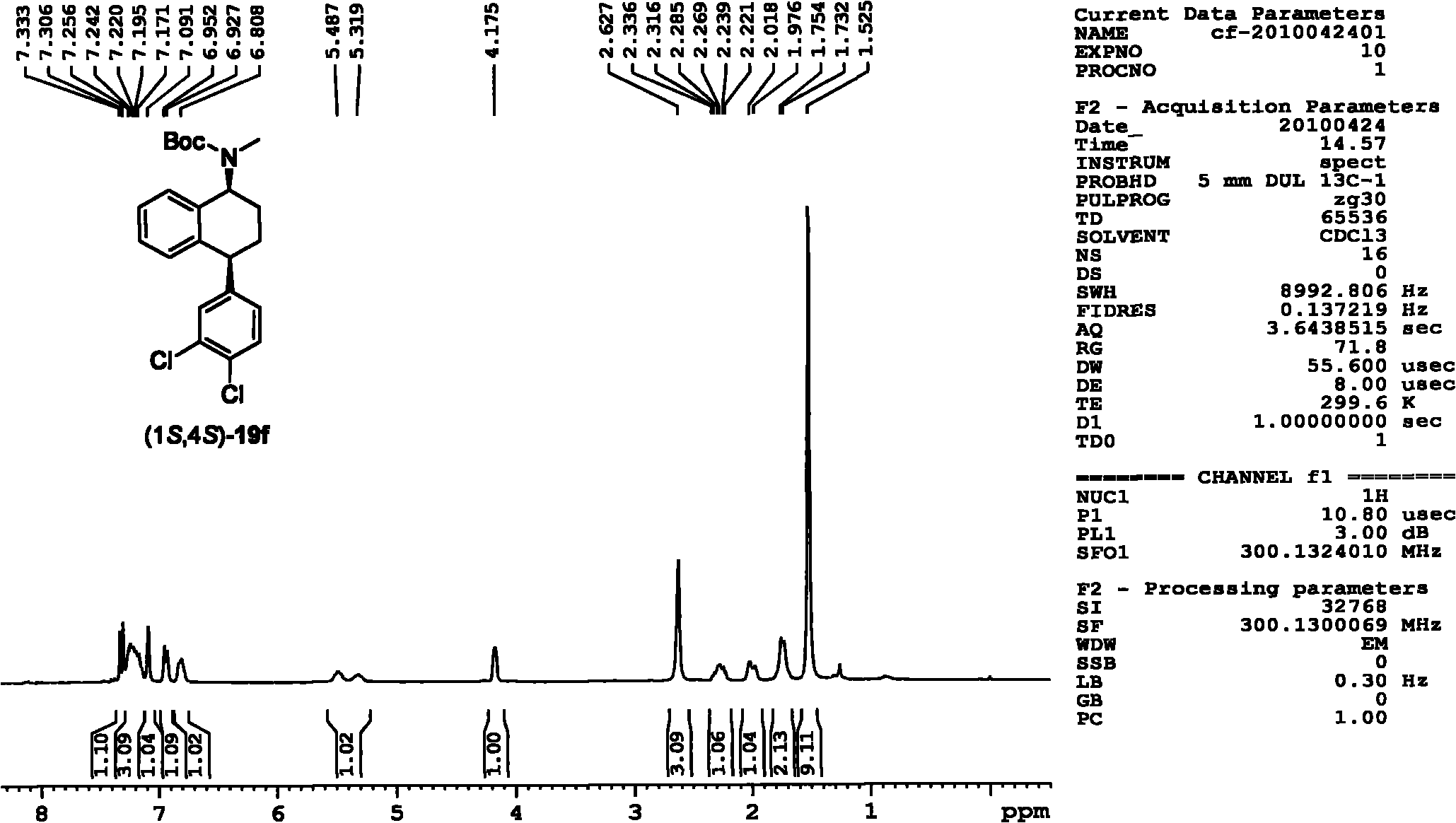
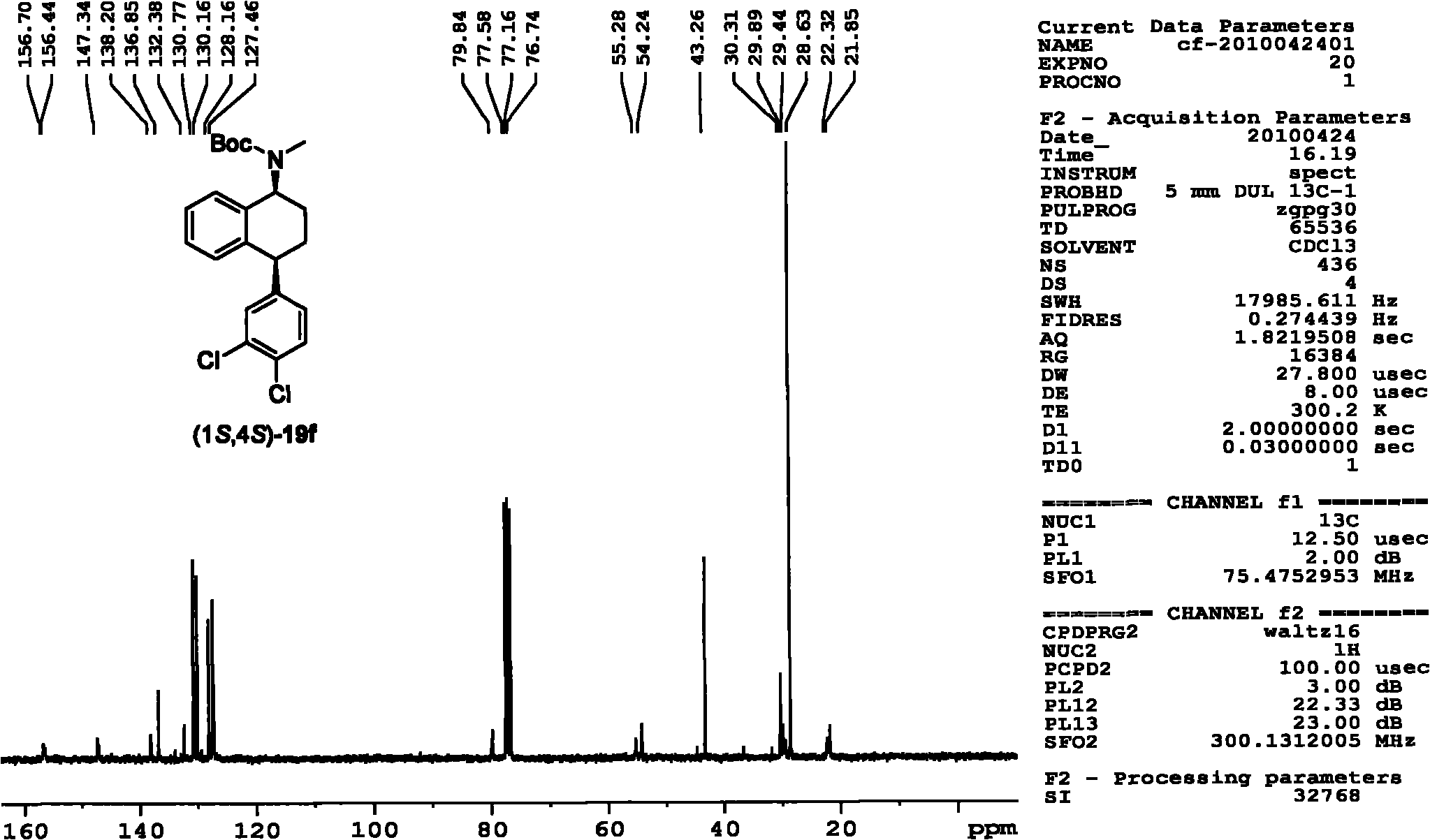
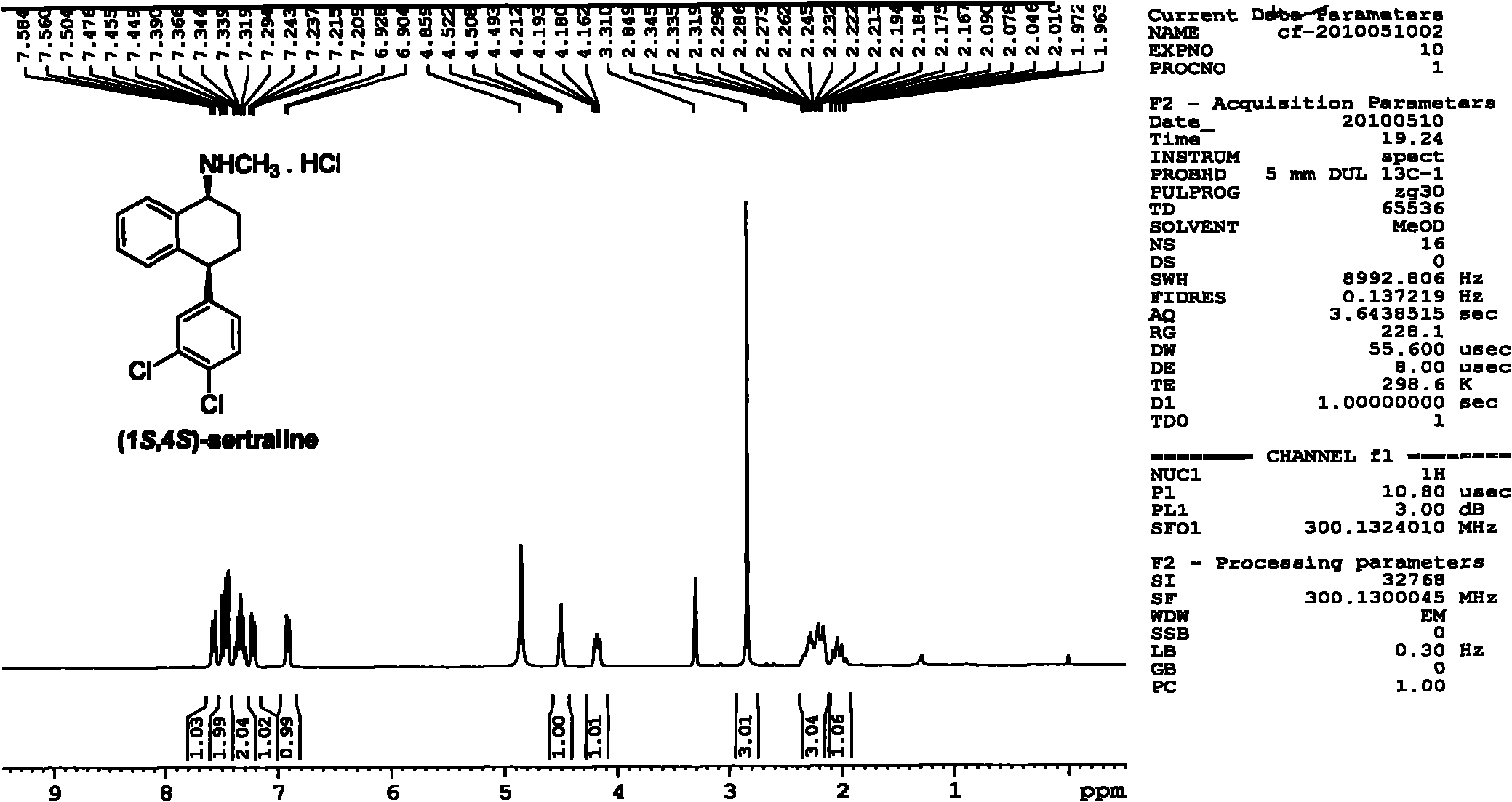
Asymmetric catalytic hydrogenation method for ketone-derived N-alkylimine
A technology for catalytic hydrogenation of alkylimines, applied in chemical instruments and methods, preparation of amino compounds, preparation of amino hydroxyl compounds, etc., can solve the problems of failing to meet the requirements of industrialization and low enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-9
[0068] Embodiment 1-9, the screening of additive and the optimization of additive dosage
[0069] Asymmetric catalytic hydrogenation of N-benzyl-phenylmethylimine from the reaction of acetophenone with benzylamine as a model reaction under the catalysts (R,R)-3a and (R,R)-4e , Carried out the screening of asymmetric catalytic reaction additives and the optimization of additive dosage, the experimental design and result of specific embodiment 1-9 are shown in Table 1.
[0070] The specific operation of the asymmetric catalytic hydrogenation reaction is as follows: the hydrogenation experiments were all carried out in a high-pressure reactor. The molar dosage is the catalyst of reaction substrate N-benzyl-phenylmethylimine 2%, the reaction substrate N-benzyl-phenylmethylimine of 0.2mmol, a certain amount of additives are dissolved in 1mL solvent , After replacing the gas with nitrogen, fill it with 50atm hydrogen, and react at 40°C for a certain period of time. In this reactio...
Embodiment 10-27
[0076] Embodiment 10-27, the screening of catalyst
[0077] Taking the asymmetric catalytic hydrogenation of N-benzyl-phenylmethylimine generated by the reaction of acetophenone and benzylamine as a model reaction, the catalysts were screened, and the catalysts used were (R, R)-3~(R , R)-15, the experimental design and results of specific examples 10-27 are shown in Table 2.
[0078]The specific operation of the asymmetric catalytic hydrogenation reaction is as follows: the hydrogenation experiments were all carried out in a high-pressure reactor. The molar dosage is the catalyst of reaction substrate N-benzyl-phenylmethylimine 2%, 0.2mmol reaction substrate N-benzyl-phenylmethylimine and 0.22mmol di-tert-butyl dicarbonate are dissolved in In 1 mL of dichloromethane, replace the gas with nitrogen, fill with 50 atm of hydrogen, and react at 40°C for 10 hours. The types of catalysts used in this reaction are shown in Table 2.
[0079] The stirring was stopped, and the reactio...
Embodiment 28-40
[0085] Embodiment 28-40, screening of asymmetric catalytic hydrogenation reaction solvent
[0086] Taking the asymmetric catalytic hydrogenation of N-benzyl-phenylmethylimine generated by the reaction of acetophenone and benzylamine as a model reaction, the screening of the reaction solvent was carried out. The experimental design and results of specific examples 28-40 are shown in the table 3.
[0087] The specific operation of the asymmetric catalytic hydrogenation reaction is as follows: the hydrogenation experiments were all carried out in a high-pressure reactor. The molar dosage is the catalyst (R, R)-4e of 2% of reaction substrate N-benzyl-phenylmethylimine, 0.2mmol reaction substrate N-benzyl-phenylmethylimine and 0.22mmol Di-tert-butyl dicarbonate was dissolved in 1 mL of reaction solvent, and after the gas was replaced with nitrogen, 50 atm of hydrogen gas was filled in, and the reaction was carried out at 40° C. for 10 hours.
[0088] Among them, the experiment nu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com