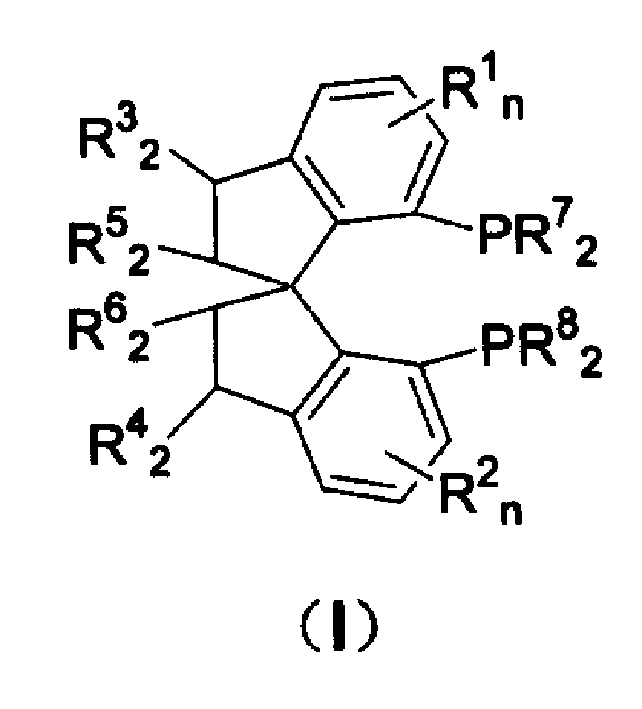
Spiro-diphosphine ligand
A technology of bisphosphine ligand and spiro ring, which is applied in the field of synthesis of chiral compounds, can solve the problems of inconvenient operation and poor stability, and achieve the effects of high stereoselectivity, high conversion number and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1 Preparation of (S)-7,7'-bis(trifluoromethanesulfonyloxy)-1,1'spirodihydroindene
[0014] Add (S)-spirobiphenol (5.0g, 19.8mmol), pyridine (7.0mL, 86.7mmol) and 100mL CH in a 250mL reaction flask 2 Cl 2 , Trifluoromethanesulfonic anhydride (8.2 mL, 43.7 mmol) was added dropwise at 0° C., and the mixture was stirred overnight at room temperature. The reaction solution was concentrated and filtered with a silica gel column to obtain (S)-7,7'-bis(trifluoromethanesulfonyloxy)-1,1'-spiroindene 9.9 g, yield: 97%; white solid, Melting point: 62-64℃; [α] D -64(c0.5CH 2 Cl 2 ).
[0015] 1 H NMR(300MHz, CDCl 3 )δ 2.35(m, 4H, CH 2 ), 3.10(m, 4H, CH 2 ), 7.15 (dd, 2H, J=1.8 and 6.6 Hz, Ar-H), 7.26-7.30 (m, 4H, Ar-H); 13 C NMR(75MHz, CDCl 3 )δ31.1, 38.6, 59.4, 115.9, 118.5, 120.1, 124.4, 129.4, 138.2, 145.8, 147.7; IR (KBr) 2958, 1622, 1579, 1466, 1405, 1214, 1144, 994, 934, 860, 830cm -1 ; MS(EI)m / z516(M + ); elemental analysis (theoretical value) C 19 H 14 F 6 O 6 S 2 : C43....
Embodiment 2
[0016] Example 2 Preparation of (S)-7-diphenylphosphono-7'-trifluoromethanesulfonyloxy-1,1'-spiroindene
[0017]Add (S)-7,7'-bis(trifluoromethanesulfonyloxy)-1,1'-spiroindene (4.0g, 7.75mmol), diphenylphosphine oxide (3.13 g, 15.5 mmol), palladium acetate (87 mg, 0.39 mmol), 1,4-diphenylphosphinobutane (dppb, 166 mg, 0.39 mmol), and 25 mL of DMSO. Diisopropylethylamine (4.1 g, 32 mmol) was added with stirring, and the mixture was heated to 100° C. to react for 6 hours. Cooled to room temperature, diluted with EtOAc, filtered, the filtrate was subjected to silica gel column chromatography after removing the solvent (eluent: petroleum ether / EtOAc=3 / 1) to obtain (S)-7-diphenylphosphono-7'- Trifluoromethanesulfonyloxy-1,1'-spiroindene 4.0 g, yield 90%; white solid, melting point: 173-175°C; [α] D -74(c0.5 CH 2 Cl 2 ).
[0018] 1 H NMR(300MHz, CDCl 3 )δ2.20-2.39(m, 4H CH 2 ), 3.08(m, 2H, CH 2 ), 3.23-3.41(m, 2H, CH 2 ), 6.21 (d, 2H, J=8.1 Hz, Ar-H), 7.14-7.20 (m, 11H, Ar-H); 31 PNMR(1...
Embodiment 3
[0019] Example 3 Preparation of (R)-7-dimethylphenylphosphono-7'-trifluoromethanesulfonyloxy-1,1'-spiroindene
[0020] The preparation method is the same as in Example 2. Yield: 81%; white solid, melting point: 222-224°C; [α] D +116(c 0.5 CH 2 Cl 2 ).
[0021] 1 H NMR(300MHz, CDCl 3 )δ2.18-2.42(s, 3H, CH 3 ), 2.32(s, 3H, CH 3 ), 2.39(s, 3H, CH 3 ), 3.05(m, 3H, CH 2 ), 3.21-3.19(m, 2H, CH 2 ), 6.24 (d, 2H, J = 8.1 Hz, Ar-H), 7.00-7.10 (m, 2H, Ar-H), 7.10-7.23 (m, 10H, Ar-H), 7.20-7.23 (m, 2H, Ar-H), 7.29(d, 2H, Ar-H); 31 P NMR(121 MHz, CDCl 3 )δ31.73(s); 13 C NMR(121 MHz, CDCl 3 ): δ21.5, 30.7, 31.8, 39.8, 40.0, 61.9, 115.6, 117.5, 123.7, 125.9, 126.2, 127.5, 127.6, 127.9, 128.2, 128.6, 128.8, 128.9, 131.3, 131.5, 131.7, 131.8, 132.2, 133.2, 133.4, 133.6, 140.9, 141.3, 144.9, 145.9, 146.1, 149.7, 152.9, 152.9; IR (KBr) 3061, 2943, 1462, 1439, 1415, 1398, 1209, 1142, 853cm -1 ; MS(EI)m / z 596(M + ); elemental analysis (theoretical value) C 32 H 28 F 3 O 4 PS: 64.16 (64.42); H4.92 (4....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com