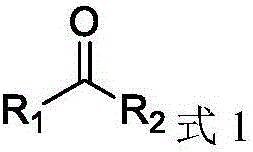
Alcohol dehydrogenase mutant, gene thereof, and application thereof in preparation of chiral diaryl alcohol
An alcohol dehydrogenase and mutant technology, which can be used in applications, genetic engineering, plant genetic improvement, etc., can solve the problems of low catalytic activity and stereoselectivity, and achieve good industrial application prospects, high catalytic activity and enantioselectivity. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Construction and screening of random mutation library of alcohol dehydrogenase KpADH
[0050] First, design primers for random mutation, as shown in Table 1 and SEQ ID No. 2 and SEQ ID No. 3 in the sequence table.
[0051] Table 1 Alcohol dehydrogenase KpADH random mutation primers
[0052]
[0053]
[0054] PCR reaction system (25μL): template 10-20ng, 10×rTaq buffer, 2.5mM dNTP mix, 100μMMnSO 4 , 500μM MgCl 2 , 1.25U rTaq polymerase (TaKaRa, Japan), add sterile distilled water to 25μL.
[0055] The error-prone PCR amplification procedure is: (1) denaturation at 94°C for 3min; (2) denaturation at 94°C for 30sec; (3) annealing at 54°C for 30sec; (4) extension at 72°C for 90s, repeat steps (2) to (4) After 30 cycles, the final extension was at 72°C for 10 minutes, and the PCR amplified product was stored at 4°C.
[0056] The amplified product and pET28a(+) vector were digested with restriction enzymes Nde I and BamH I, respectively, to form complementary sticky ends. 4 ...
Embodiment 2
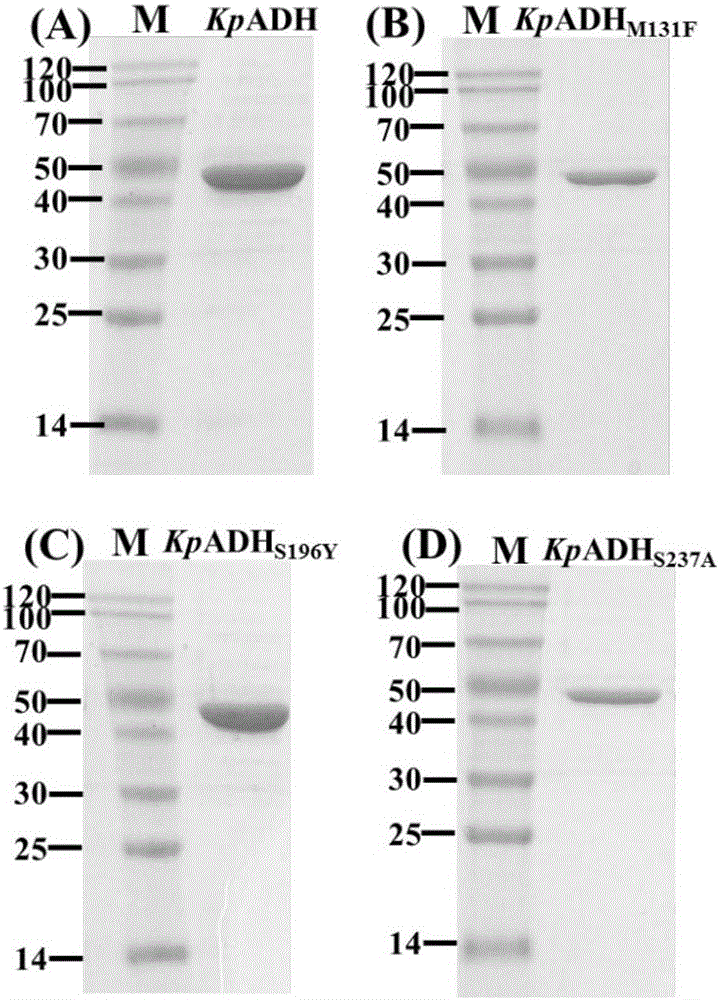
[0060] Example 2 Expression and purification of alcohol dehydrogenase mutants
[0061] Transform the mutants with increased activity into fresh LB culture at 2% transfer volume and cultivate to OD 600 When it reaches 0.6-0.8, add 0.2mM IPTG, 30℃induction culture for 6 hours, 4℃, 8000r / min centrifugation for 10min to collect the bacteria. Suspend the collected bacteria in potassium phosphate buffer (100mM, pH 6.0), and ultrasonically disrupt
[0062] The column used for purification is the affinity column HisTrap FF crude (nickel column), which uses the histidine tag on the recombinant protein for affinity binding. First use solution A to equilibrate the nickel column, load the crude enzyme solution, continue to use solution A to elute the breakthrough peak, and use solution B (20mmol·L -1 Sodium phosphate, 500mmol·L -1 NaCl, 1000mmol·L -1 Imidazole, pH 7.4) was subjected to gradient elution, and the recombinant protein bound to the nickel column was eluted to obtain a recombinant a...
Embodiment 3
[0063] Example 3 Kinetics and selectivity analysis of alcohol dehydrogenase mutants
[0064] Determine the activity of KpADH at different substrate concentrations and coenzyme concentrations, and make a double reciprocal curve based on the reciprocal of the activity and substrate concentration to calculate the kinetic parameters.
[0065] Table 2 shows the k of wild-type alcohol dehydrogenase KpADH to CPMK cat / K m And ee are respectively 16.8L·s -1 ·Mmol -1 And 82.0%, mutant enzyme KpADH M131F , KpADH S196Y And KpADH S237A K for CPMK cat / K m And ee are respectively 17.8L·s -1 ·Mmol -1 And 82.1%, 19.3L·s -1 ·Mmol -1 And 74.7%, 79.2L·s -1 ·Mmol -1 And 96.1%. Mutant enzyme KpADH S237A Shows the highest catalytic activity and stereoselectivity, the k of CPMK cat / K m It is 4.71 times that of the wild type.
[0066] Table 2 Kinetic parameters and stereoselectivity of alcohol dehydrogenase mutants
[0067]
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com