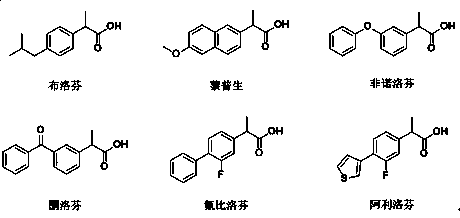
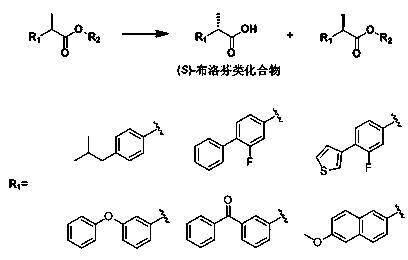
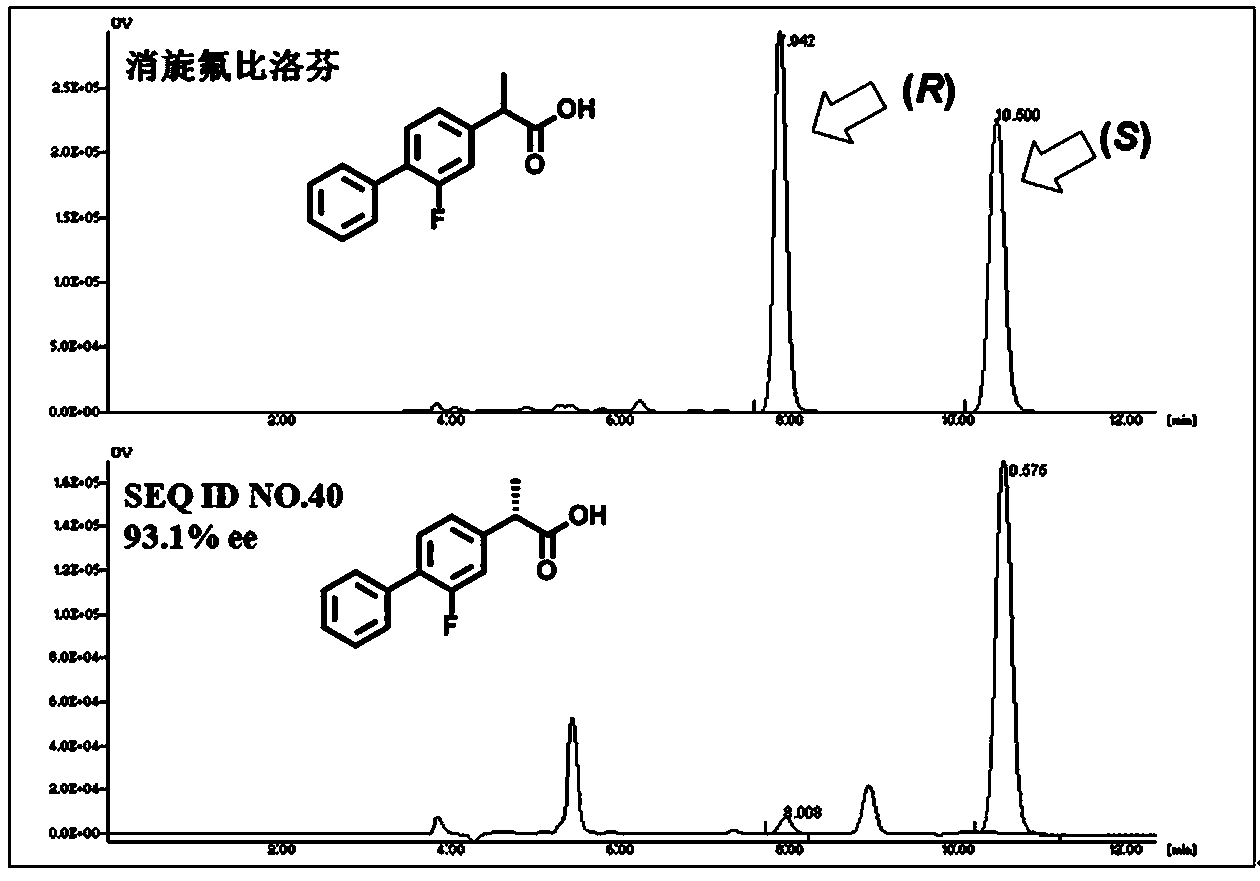
Lipase mutant and uses thereof
A mutant and lipase technology, applied in the field of lipase mutants and their applications, can solve the problems of low enantioselectivity and achieve the effect of improving enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Extraction of Genomic DNA from Candida Antarctica (cgmcc 2.2366) and Cloning of Wild Type Lipase B Gene
[0050] After culturing Candida antarctica cgmcc 2.2366 with YPD liquid overnight, the fermentation broth was centrifuged in a 1mL centrifuge tube at 3000r / min for 5min, and the supernatant was discarded to collect the cells, which could be repeated several times to obtain a sufficient amount of cells; Resuspend the DNA lysate, add 0.4g acid-washed glass beads, then add 200μL phenol: chloroform: isoamyl alcohol, cover the tube cap tightly to prevent phenol from leaking when vortexing; c) Vortex at high speed for 3min; d ) Centrifuge at 12000 r / min for 5 minutes, transfer the supernatant (about 200 μL) to a new centrifuge tube; e) Add 700 to the supernatant and add pre-cooled absolute ethanol, and place it in anhydrous at -20°C for 1 hour, 12000 r / min Centrifuge for 15 minutes to precipitate DNA, pour off ethanol, use 800 μL ethanol for precipitation, susp...
Embodiment 2
[0051] Example 2 Polymerase Chain Reaction (PCR)
[0052] Using the extracted Candida Antarctica genomic DNA as a template for PCR reaction, the reaction system is as follows:
[0053] TaKaRaTaq (5 U / μL) 0.25 μL 10×PCR Buffer (Mg 2+ Free) 5 μL MgCl 2 (25mM) 3μL dNTP mixture (2.5 mM each) 4μL template DNA genome 1μL Primer 1 / 3 (10μM) 2μL Primer 2 / 4 (10 μM) 2μL Sterile distilled water Make up to 50 μL
[0054] Amplification program: 94°C: 10 Min, (94°C: 30s, 45°C: 30s, 72°C: 30s) 35 cycles, 72°C, 10min.
[0055] Primer 1: CALB- Eco R I-f: 5Cal B- GAATTCC TACCTTCCGGTTCGGACC-3
[0056] Primer 2: CALB- not I-r: 5CalB-Not I-rC GCGGCC GCTCAGGGGGTGACGATGCC-3;
[0057] Restriction endonuclease cut sites are underlined;
[0058] The DNA fragments amplified by PCR were purified using a gel extraction kit. Containing the pPICZα A plasmid E. coli Top 10' was cultured overnight in LB liquid medium at 37°C...
Embodiment 3
[0061] Example 3 E. coli Preparation and transformation of DH5α competent cells
[0062] a) Take 0.4mL from the seed culture medium and inoculate it into 20mL LB liquid medium for 3h; b) 3000r / min, enrich 2mL of bacteria in 1.5mLEP tube twice in 5min, discard the supernatant; c) add 100 Discard the ice-cold TSS solution, re-suspend the bacteria, and ice-bath for 30 minutes; d) Add 20 μL of connection solution (empty plasmid pPICZα A enzyme-digested fragment, target fragment enzyme-digested fragment and connection solution) and gently swirl to mix well, and ice-bath for 30 minutes; e ) heat shock at 42°C for 60s, ice bath for 2min, and add 600 μL LB liquid medium. Cultivate at 37°C and shake at 150r / min for 1h; f) Take 150μL of each and spread on LLB (ZeocinTM) resistance plate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com