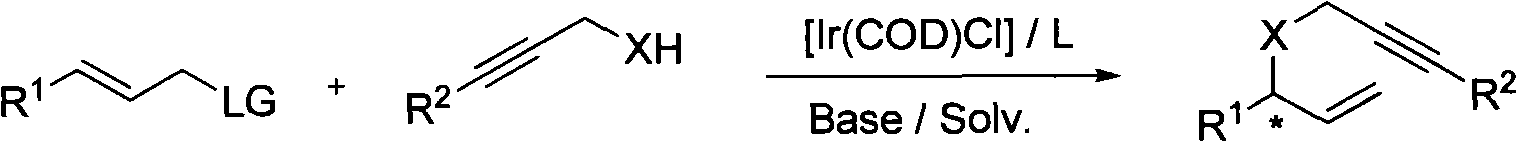Method for synthesizing 1,6-eneyne compounds
A compound, enyne technology, applied in 1 field, can solve the problem of less research and achieve the effect of simple operation, high regioselectivity and high enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
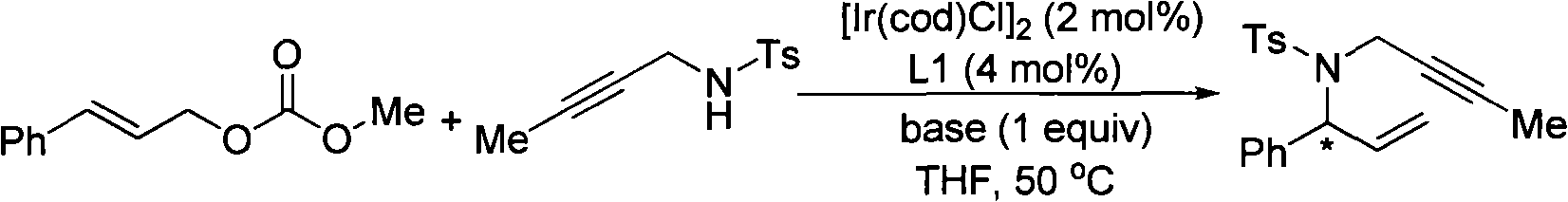
[0021] Embodiment 1: Allyl carbonate and propargyl nucleophile under metal iridium complex catalyzed the research of the base of asymmetric allylation reaction:
[0022]
[0023] Wherein, mol means mole, and base means base.
[0024]
[0025]Among them, THF is tetrahydrofuran, toluene is toluene, dioxane is dioxane, DME is dimethylethylene glycol, DCM is dichloromethane, DBU is 1,8-diazabicyclo[5,4,0] Undec-7-ene, DABCO triethylenediamine.
Embodiment 2
[0026] Embodiment 2: the research of the solvent that allyl carbonate and propargyl nucleophile take place asymmetric allylation reaction under metal iridium complex catalysis:
[0027]
[0028]
[0029] Among them, THF is tetrahydrofuran, toluene is toluene, dioxane is dioxane, DME is dimethylethylene ether, DCM is dichloromethane, and DABCO triethylenediamine.
Embodiment 3
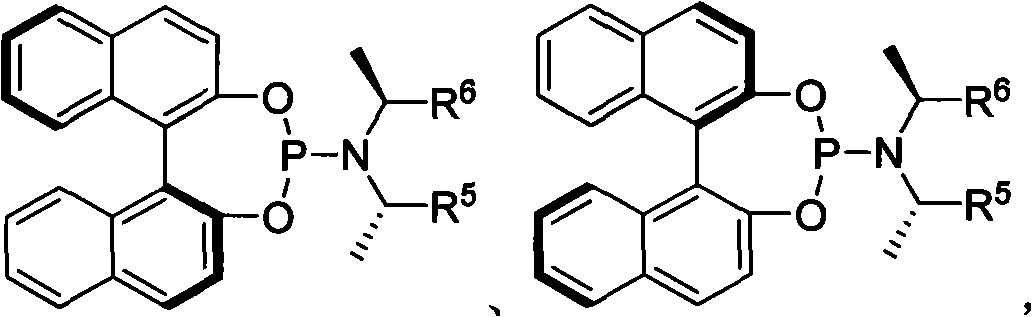
[0030] Embodiment 3: Allyl carbonate and propargyl nucleophile under metal iridium complex catalyzed the research of the part of asymmetric allylation reaction:
[0031]
[0032]
[0033] (S, S, S a ) (S,S,R a )-L4 (S, S, S a )-L5
[0034] L1R=Ph
[0035] L2R=2-MeO-C 6 h 4
[0036] L3R=2-naphthyl
[0037]
[0038] Where Ph is phenyl, Naphthyl is naphthyl and MeO is methoxy.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com