Spiro phosphine-oxazoline and preparation method and application thereof
A technology of oxazoline and spiro ring, applied in the field of new spiro phosphine-oxazoline and preparation, can solve the problems of reducing the scope of application of SIPHOX ligand substrates and high cost of SIPHOX ligand synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
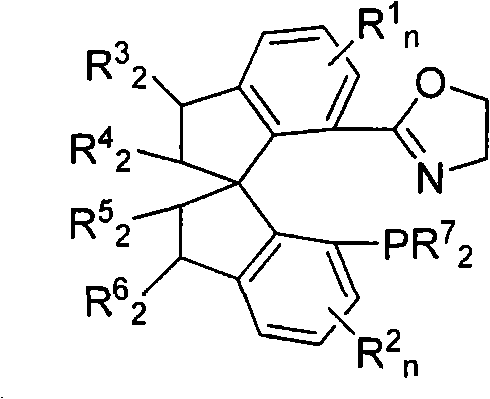
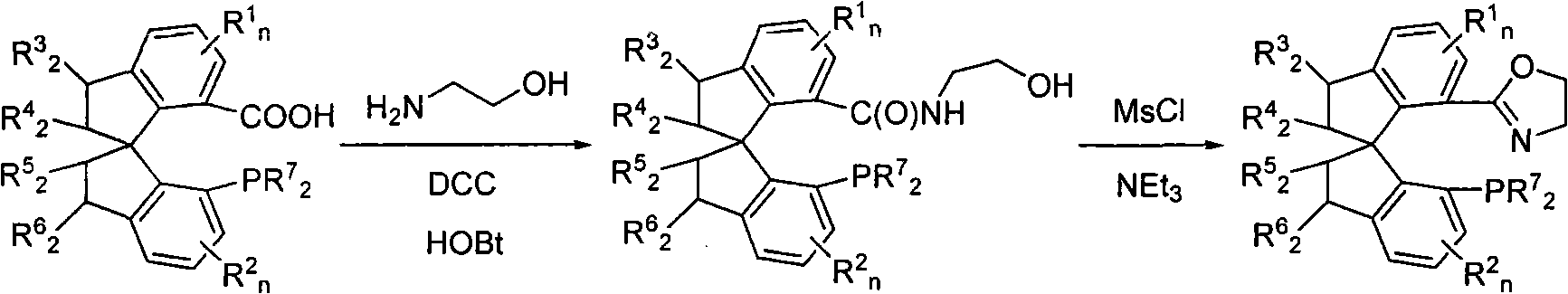
[0040] Embodiment 1: (S a Preparation of )-N-hydroxyethyl-7'-bis(3,5-di-tert-butylphenyl)phosphino-1,1'-spirodihydroindene-7-carboxamide
[0041]
[0042] Weigh (S a )-7-bis(3,5-di-tert-butylphenyl)phosphino-7'-carboxy-1,1'-spirodihydroindane 1 (1.0g, 1.48mmol), aminoethanol (284mg, 4.65mmol ), 1-hydroxybenzotriazole (HOBt, 504mg, 3.29mmol) and dicyclohexylcarboimide (DCC, 881mg, 4.27mmol). Added redistilled tetrahydrofuran (THF, 80mL) under ice-water cooling, after the addition, the reaction was naturally raised to room temperature and stirred, and a large amount of white precipitates formed in the system. TLC followed the reaction until conversion was complete. Then add 5g of silica gel, rotary evaporation precipitation, dry column, with sherwood oil / ethyl acetate mixed solvent (v / v=1: 1) as eluent to carry out silica gel column chromatography, to obtain compound (S a )-N-Hydroxyethyl-7'-bis(3,5-di-tert-butylphenyl)phosphino-1,1'-spiroindane-7-carboxamide 2 (660 mg, 6...
Embodiment 2
[0045] Embodiment 2: (S a Preparation of )-7-(4,5-dihydrooxazol-2-yl)-7'-bis(3,5-di-tert-butylphenyl)phosphino-1,1'-spiroindene
[0046]
[0047] In a 100mL Schlenk reaction flask equipped with electromagnetic stirring, weigh (S a )-N-hydroxyethyl-7'-bis(3,5-di-tert-butylphenyl)phosphino-1,1'-spirodihydroindane-7-carboxamide 2 (660mg, 0.92mmol) and 4 - Dimethylaminopyridine (DMAP, 5mg, 0.041mmol), the vacuum line was replaced with a nitrogen atmosphere, and 60mL of degassed dichloromethane was added and stirred evenly. Under cooling in an ice-water bath, 0.28 mL of triethylamine and methanesulfonyl chloride (MsCl, 105 μL, 1.36 mmol) were sequentially added, kept stirring for 30 minutes, and then 1.20 mL of triethylamine was added, and allowed to rise to room temperature and stirred overnight. TLC followed the reaction until conversion was complete. Add 5g of silica gel to the system to quench the reaction, and then directly dry-load the column after vacuum precipitation,...
Embodiment 3
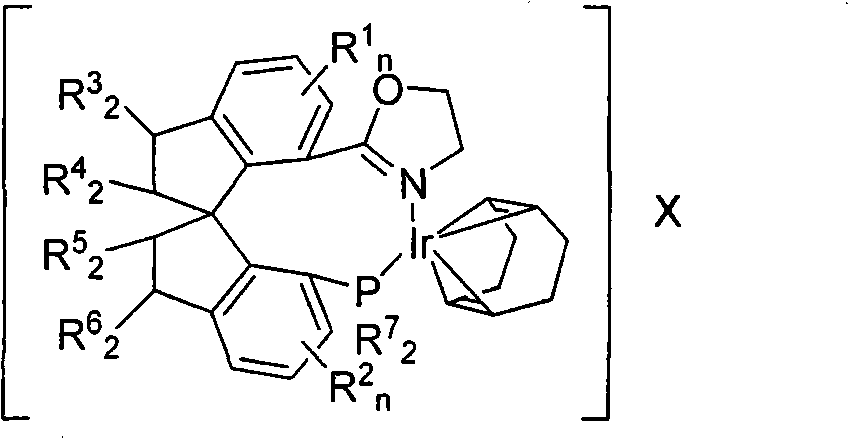
[0050] Embodiment 3: [(S a - Preparation of DTB-SIPHOX)Ir(COD)]BARF
[0051]
[0052] Weigh the ligand (S a )-7-(4,5-dihydrooxazol-2-yl)-7'-bis(3,5-di-tert-butylphenyl)phosphino-1,1'-spirodihydroindene 3 (60mg , 0.085mmol), [Ir(COD)Cl] 2 (32mg, 0.047mmol) and NaBARF (100mg, 0.107mmol) in a 15mL Schlenk reaction flask, after taking it out, add freshly distilled dichloromethane (2mL) with a syringe, heat and stir in a water bath at 45°C for 1 hour, and monitor by thin-layer chromatography For the reaction situation, stop heating when the ligands are completely complexed, and allow the system to cool down to room temperature naturally. After rotary evaporation precipitation, residue column chromatography can be obtained [(S a -DTB-SIPHOX)Ir(COD)]BARF4 (132 mg, 82%). The product was an orange foamy solid. Mp 196°C; [α] D 21 +122.6(c 0.5, CH 2 Cl 2 ); 1 H NMR (300MHz, CDCl 3 )δ7.65 (brs, 9H, Ar-H), 7.50-7.35 (m, 8H, Ar-H), 7.27-7.24 (m, 1H, Ar-H), 7.18-7.07 (m, 4H, A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com