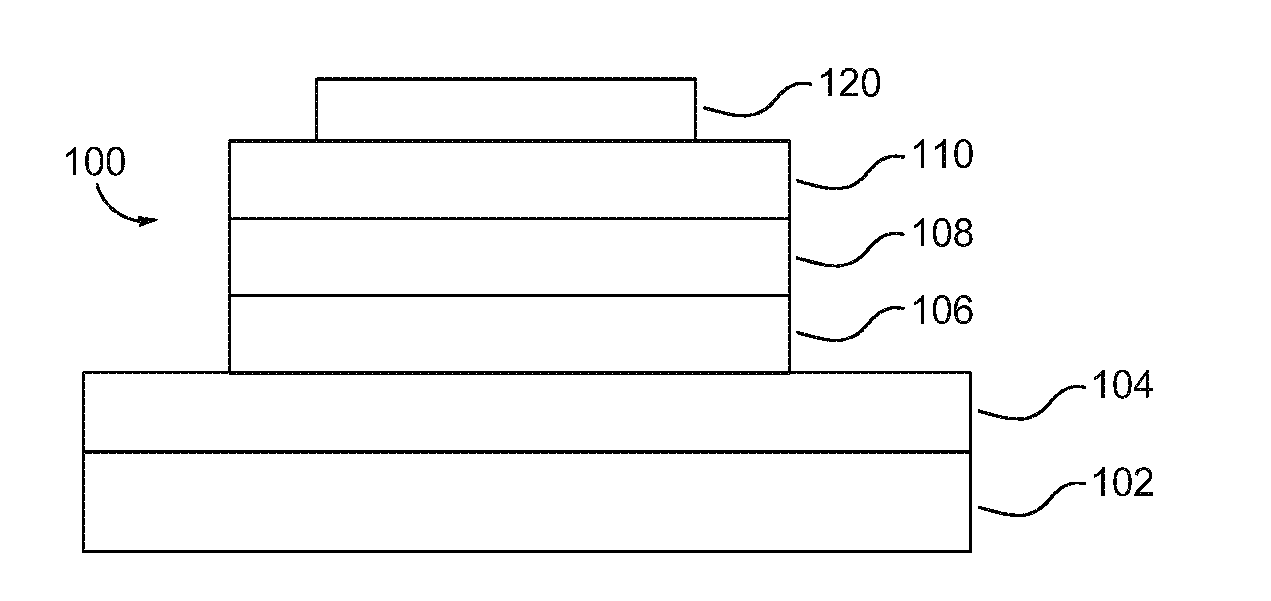
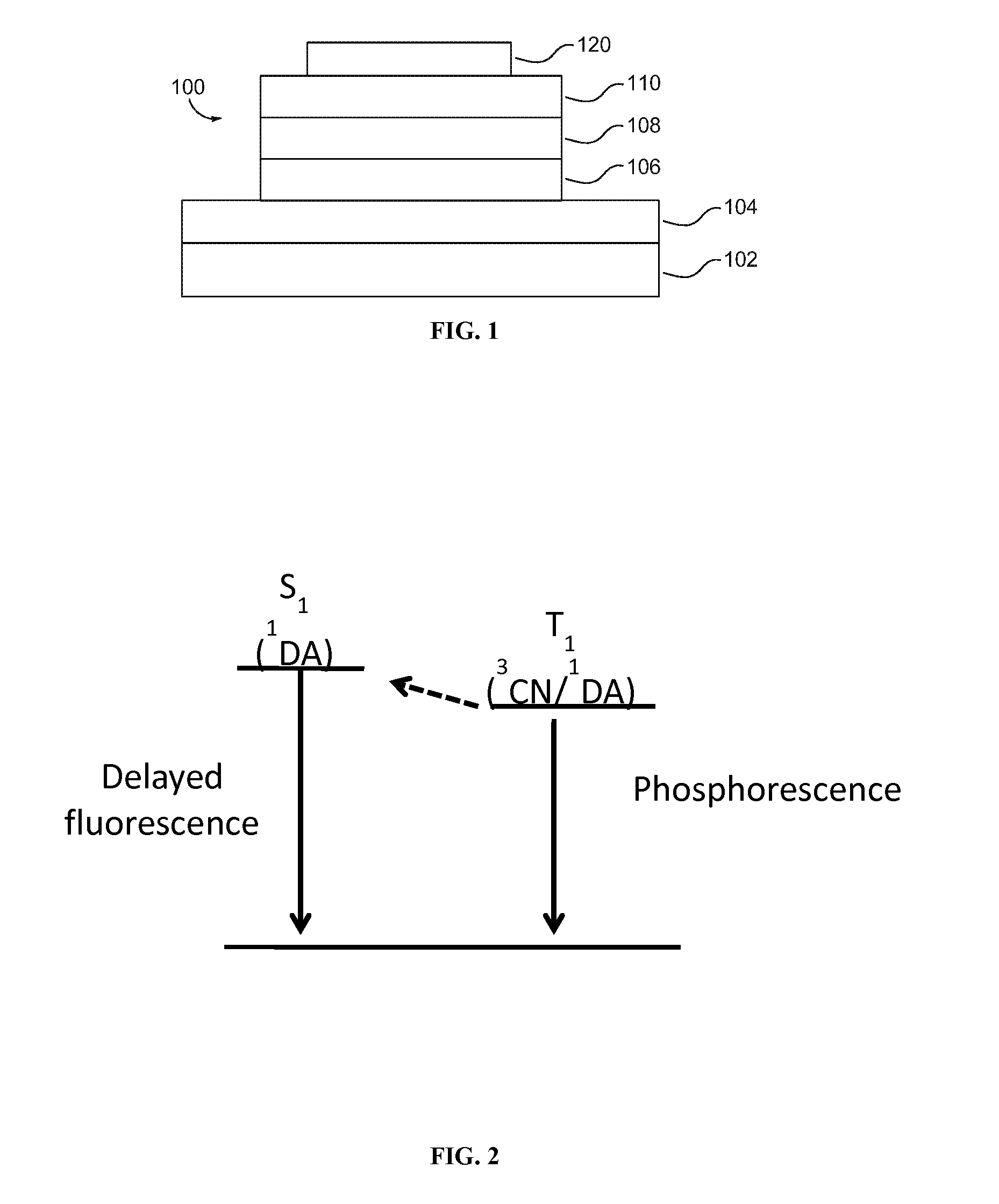
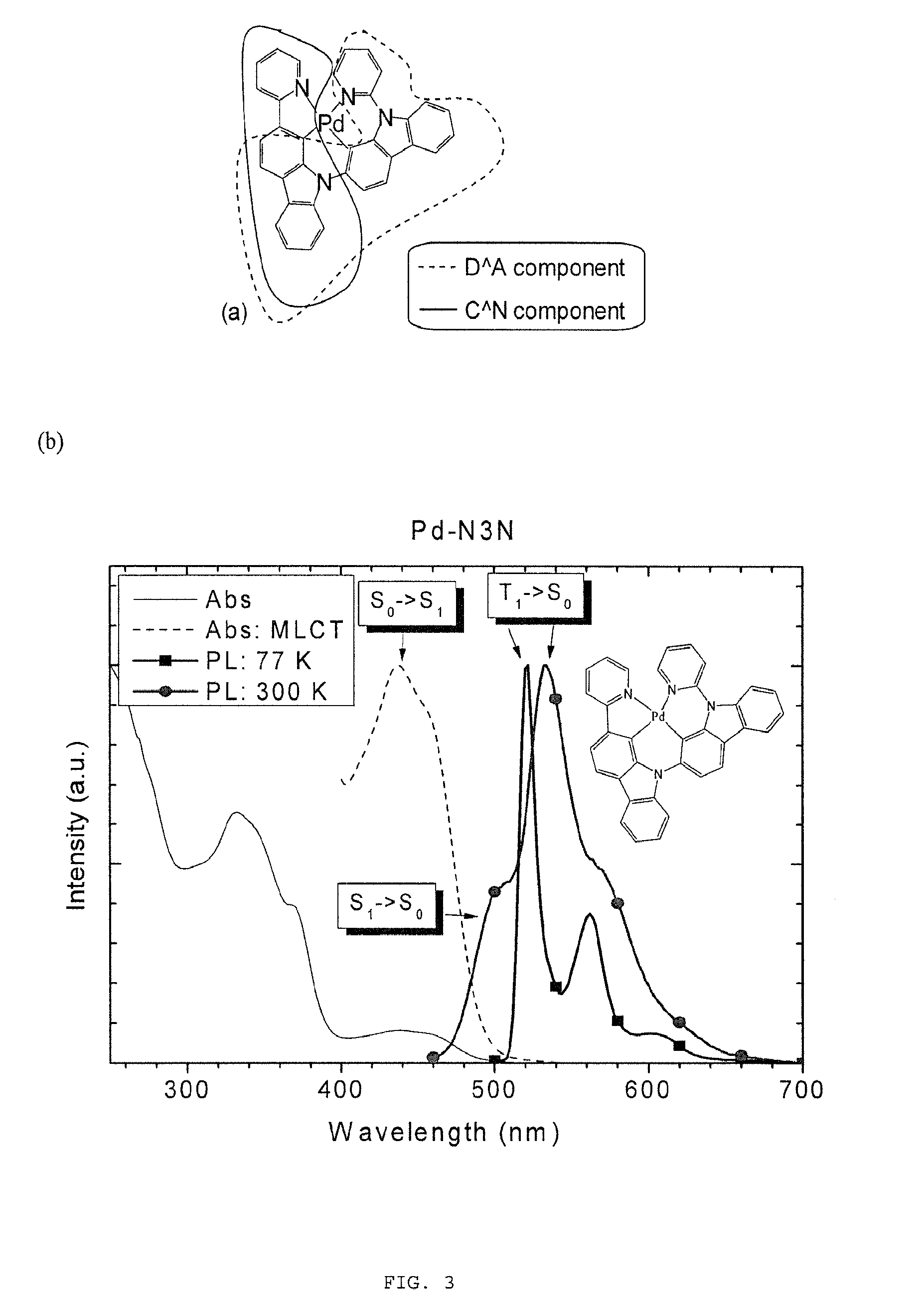
Metal complexes, methods, and uses thereof
a technology of metal complexes and complexes, applied in the field of metal complexes or compounds, can solve the problems of poor processing ability, and inability to meet the requirements of the mission or absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of 4′-bromo-2-nitrobiphenyl
[0156]
[0157]Under a nitrogen atmosphere, 20 mL of water was heated to 60° C. and 125 mmol of 2-nitrobyphenyl was added and stirred for 30 minutes before 6.3 mmol of iron trichloride was added and stirred for 30 minutes further. 140 mmol was added drop wise over 40 minutes and allowed to stir overnight before setting to reflux for 4 hours. After cooling, residual bromine was removed by washing with a sodium bisulfate solution. The organic residue was then washed with concentrated sodium hydroxide, and then twice with water. The organic portion was separated and dissolved in dichloromethane before being dried with magnesium sulfate. The solution was concentrated under reduced pressure, subjected to flash column chromatography of silica with dichloromethane as the eluent, and concentrated again under reduced pressure. 4′-bromo-2-nitrobiphenyl was collected by recrystallization from methanol in 50% yield.
example 2
Synthesis of 2-bromo-9H-carbazole
[0158]
[0159]Under a nitrogen atmosphere, 100 mmol of 4′-bromo-2-nitrobiphenyl was set to reflux overnight in stirring tirethylphosphite. After cooling, the triethylphosphite was distilled off and 2-bromo-9H-carbazole was isolated by recrystallization from methanol and further purified by train sublimation, resulting in a 65% yield.
example 3
Synthesis of 2-bromo-9-(pyridin-2-yl)-9H-carbazole
[0160]
[0161]Under a nitrogen atmosphere, 10 mmol of 2-bromo-9H-carbazole, 10 mmol of 2-bromopyridine, 1 mmol of copper(I)iodide, 25 mmol of potassium carbonate, and 2 mmol of L-proline were combined in stirring degassed dimethyl sulfoxide. The mixture was heated to 90° C. for 3 days before being cooled and separated between dichloromethane and water. The water layer was washed twice with dichloromethane and the organics were combined and washed once with brine. The organic fraction was dried with magnesium sulfate and concentrated under reduced pressure and subjected to column chromatography of silica with dichloromethane as the eluent. After concentrating under reduced pressure, 2-bromo-9-(pyridin-2-yl)-9H-carbazole was isolated in a 70% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com