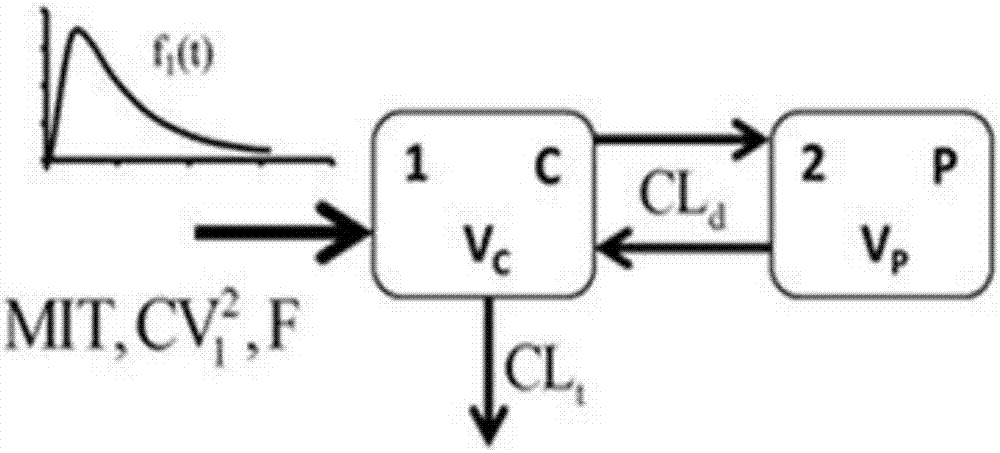
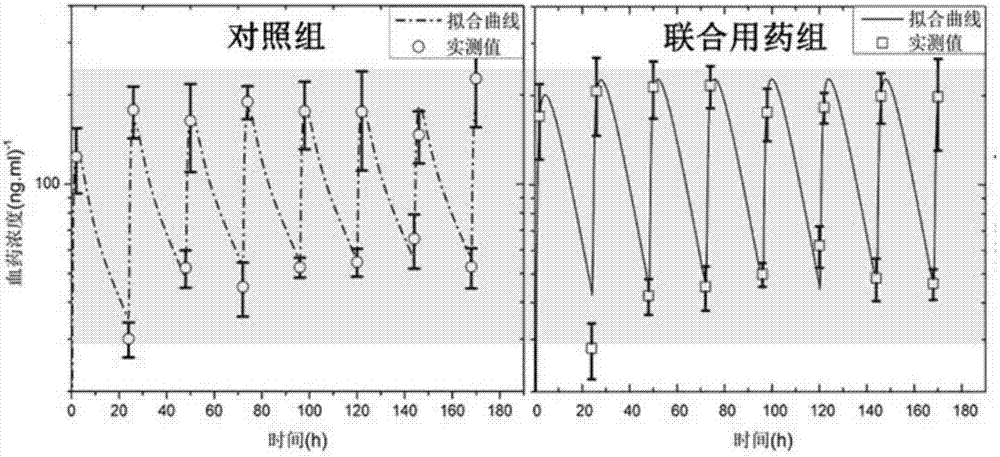
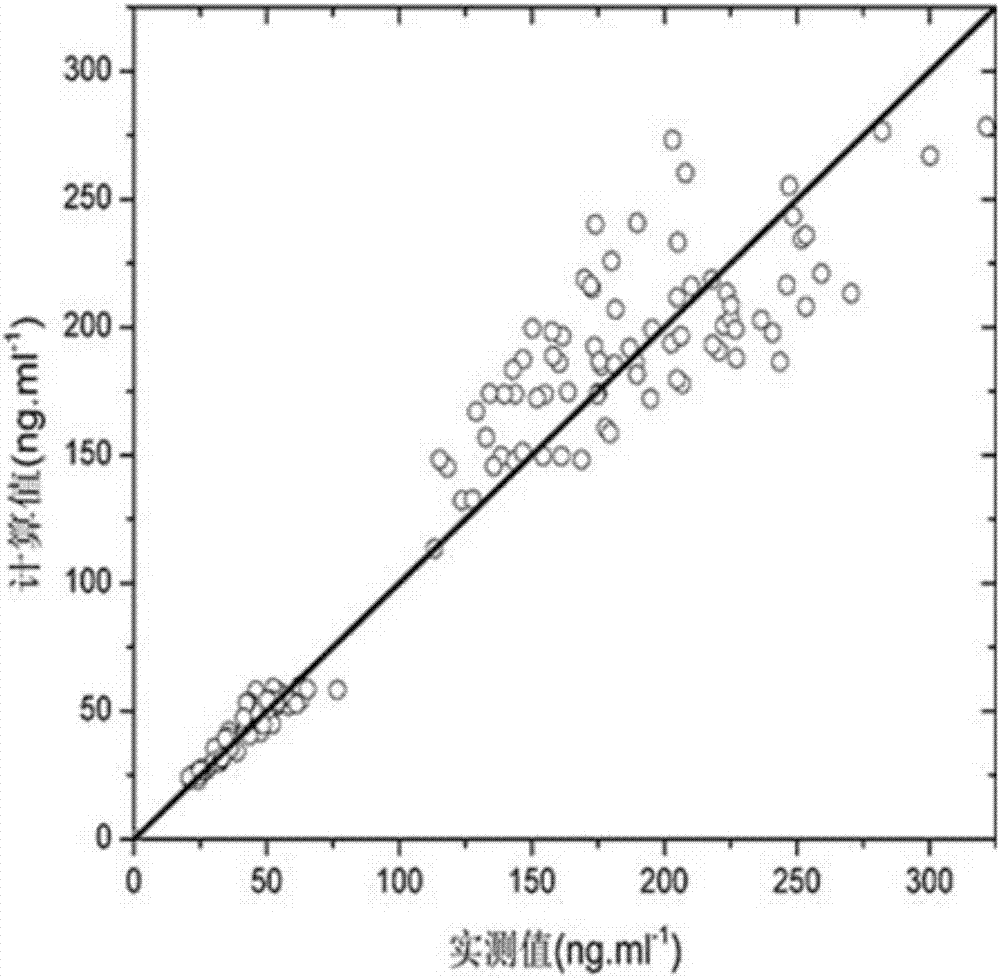
Method for evaluating bioavailability in combined application of fuke qianjin tablets and antibiotics
A technology of gynecological Qianjin tablet and combined application, applied in biological testing, scientific instruments, material inspection products, etc., can solve the problems of unguaranteed reliability and practicability, complex ingredients, and many medicinal flavors.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 1. Reagents and Materials
[0045] 1.1 Drugs and reagents
[0046] Fukeqianjin Tablets (provided by Zhuzhou Qianjin Pharmaceutical Co., Ltd.); Zithromax Azithromycin Tablets (Zithromax, Pfizer Pharmaceutical Co., Ltd.); Acetonitrile (chromatographically pure, American Fisher Company); water is Watsons distilled water; Azithromycin, Roxithromycin Standard products were purchased from China National Institutes for Food and Drug Control; Staphylococcus aureus (ATCC25923), Escherichia coli (ATCC25922) were purchased from China National Culture Bank, M-H (B) medium (OXOID company); normal saline ( Sichuan Kelun Pharmaceutical Co., Ltd.).
[0047] 1.2 Instruments
[0048] Shimadzu HPLC-MS 2020 (ESI interface), chromatographic workstation (Lab solution 5.5) (Shimadzu, Japan); QL-901 vortex mixer (Jiangsu Haimen Qilin Medical Instrument Factory); H2050R desktop high-speed refrigerated centrifuge (Xiangyi Centrifuge Instrument Co., Ltd.); CP-124S electronic balance (Beijing...
Embodiment 2
[0086] The optimization experiment of embodiment 2 modeling method
[0087] In this embodiment, the following typical experiments on modeling are selected for illustration among a large number of experiments:
[0088] (1) Material
[0089] Animals: female SPF grade SD rats, 220g±20g, purchased from the Institute of Experimental Animals, Sichuan Academy of Medical Sciences, experimental animal production license number: SCXK (Sichuan) 2008-24. 24.
[0090] Experimental strains: Staphylococcus aureus (ATCC25923), Escherichia coli (ATCC25922) were purchased from China National Culture Bank. Suspend the lyophilized powder of the tested strains in sterile M-H(B) medium and spread evenly on the surface of the sterile M-H(A) plate, incubate overnight at 35°C for revival, after 18 hours a lawn grows on the surface of the plate, select the ones with good growth Single bacterial mass, re-smear on the M-H(A) slant, incubate under the same conditions for 18h, seal, and store in refriger...
Embodiment 3
[0111] The optimization test of embodiment 3 detection conditions
[0112] (1)HPLC-ECD
[0113] Instruments: LC-30AUPLC (Shimadzu, Japan), AntecDecadeIII electrochemical detector (Antec, Netherlands), chromatography workstation (labsolution5.5); QL-901 vortex mixer (Shanghai Huxi Analytical Instrument Co., Ltd.); MTN-2800W Type nitrogen blowing instrument (Tianjin Autosines Instrument Co., Ltd.); ultra-low temperature refrigerator (FIS13-990-16THERMOSCIENTIFIC); H2050R desktop refrigerated centrifuge (Xiangyi Centrifuge Instrument Co., Ltd.); electronic balance (Beijing Sartorius Co., Ltd. )Wait.
[0114] Animals: SPF grade SD rats, 220g±20g, purchased from the Institute of Experimental Animals, Sichuan Academy of Medical Sciences, experimental animal production license number: SCXK (Sichuan) 2008-24.
[0115] Drugs and reagents: Zithromax azithromycin tablets (Zithromax, Pfizer Pharmaceutical Co., Ltd.); acetonitrile, methanol (chromatographically pure Fisher Scientific); f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com