Dosing methods for treating autoimmune diseases using a taci-ig fusion protein such as atacicept
The technology of a medicine and a composition is applied in the application field of a TACI-Ig fusion protein such as ATACICEPT for preparing a medicine for the treatment of lupus erythematosus, and can solve problems such as renal complications, side effects, and seriousness that cannot significantly affect disease progression.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
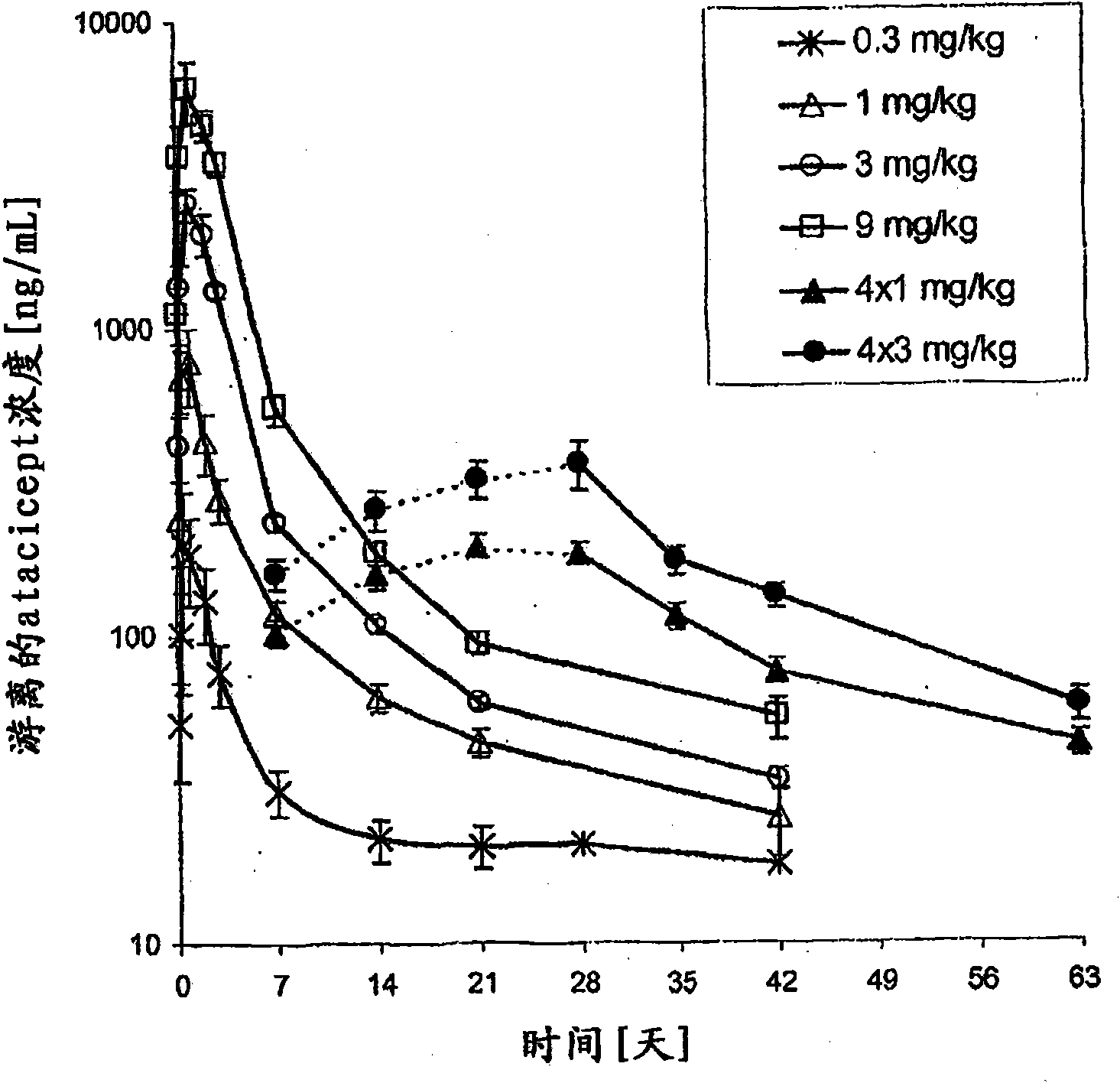
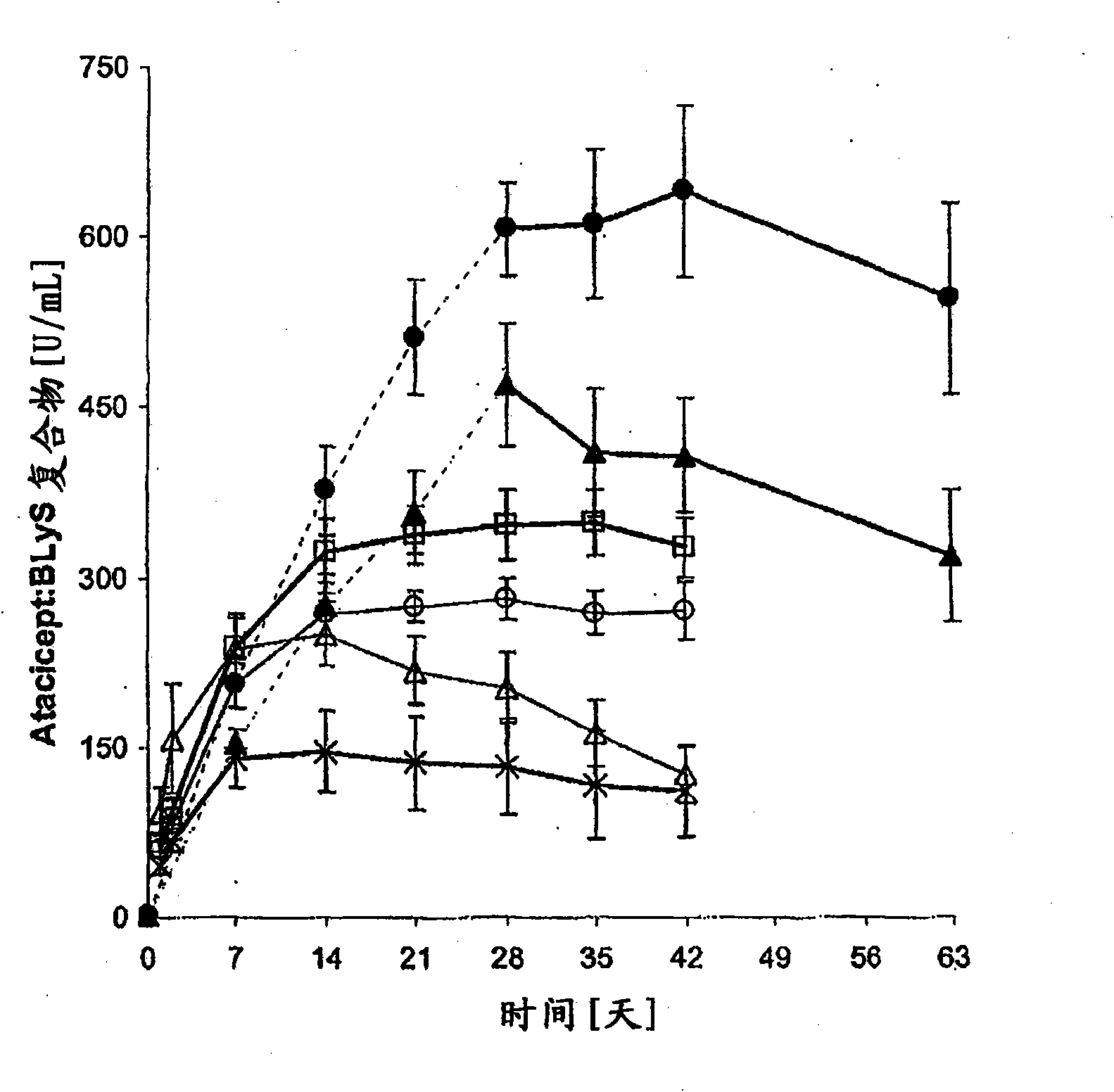
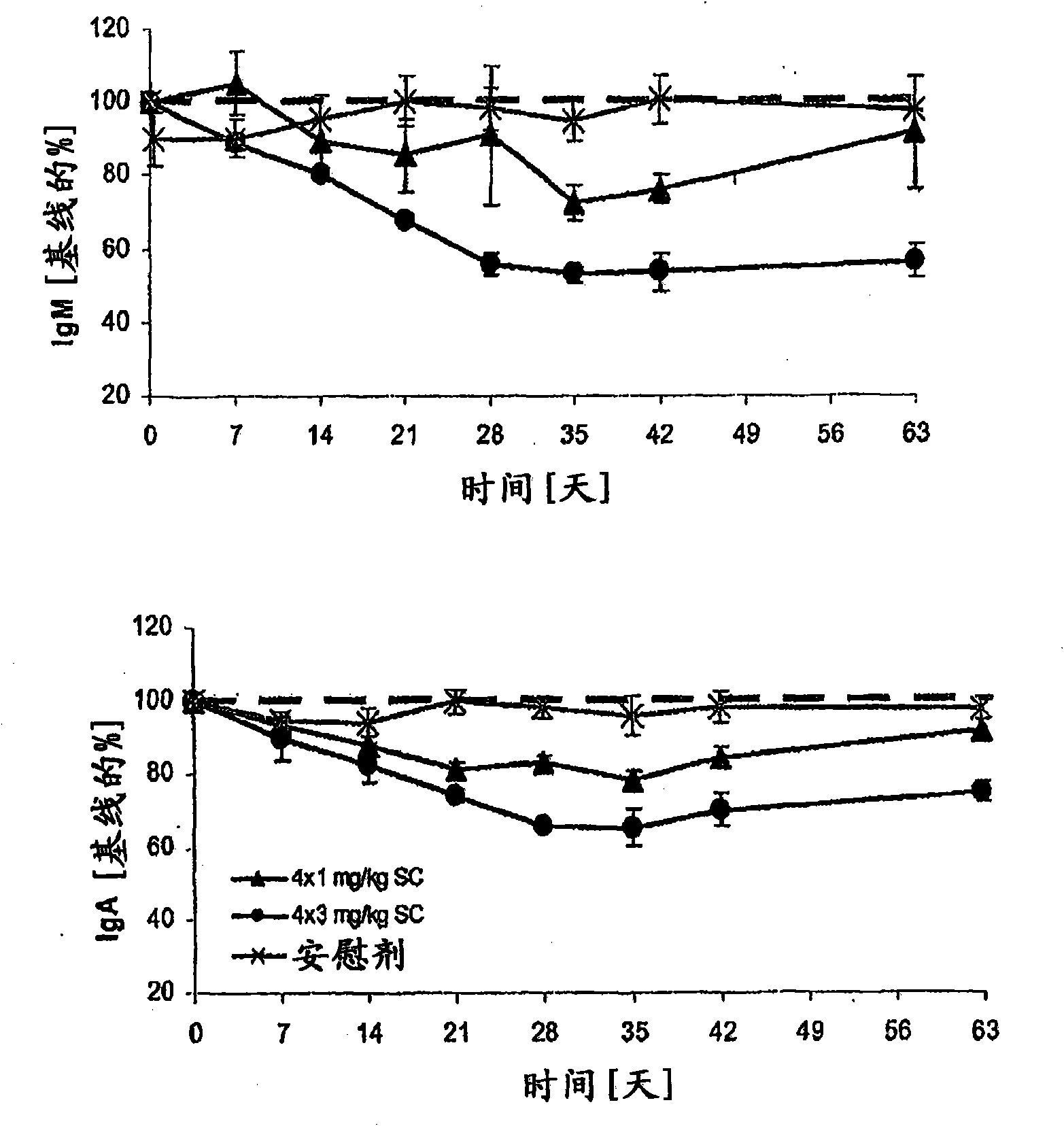
[0073] Example 1 - Subcutaneous Administration of Atacicept
[0074] This phase Ib, double-blind, placebo-controlled, dose-escalation trial included six arms (n = 8 each, except arm 5, n = 7) treated with atacicept or placebo (3:1 ratio). patient. Groups 1-4 received a single subcutaneous dose of placebo, or atacicept at 0.3, 1, 3 or 9 mg / kg. Groups 5 and 6 received 4 weekly doses of placebo, or atacicept at 1 or 3 mg / kg (see Table 1). Patients were then maintained for 6 weeks (Groups 1-4) or 9 weeks (Groups 5 and 6). Measured structures include: (i) systemic and local tolerability of atacicept; (ii) frequency of adverse events (AEs); (iii) pharmacokinetics and pharmacodynamics of atacicept, including effects on lymphocyte subsets and Effect of Ig levels; and (iv) Measurement of SLE disease activity.
[0075] Patients with mild to moderate SLE participated in the trial. The biological activity of atacicept was demonstrated by a dose-dependent reduction in immunoglobulin l...
Embodiment 2
[0089] Intravenous administration of embodiment 2-atacicept
[0090]This Phase Ib, double-blind, placebo-controlled, dose-escalation trial included 4 groups (n=6 each) of patients treated with atacicept or placebo (3:1 ratio). Groups 1-3 received a single dose of placebo, 3, 9 or 18 mg / kg of atacicept. Group 4 received two doses of placebo or 9 mg / kg atacicept, the second dose being given three weeks after the initial dose (see Table 1). Outcomes measured included: (i) systemic and local tolerability of intravenous atacicept; (ii) frequency of adverse events (AEs); (iii) pharmacokinetics and pharmacodynamics of intravenous atacicept, including effects on lymphatic Effects of cell subpopulations and Ig levels; and (iv) measurement of SLE disease activity. Subjects were evaluated at 6 weeks (Cohorts 1-3) or 9 weeks (Cohort 4); subjects in Cohorts 3 and 4 returned on study days 84 and 120 for PK and biomarker sampling . Serum PK markers were sampled as follows: (i) For single...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com