Degarelix acetate injectable sustained release implant
A technology of degarelix acetate and sustained-release implants, which is applied in aerosol delivery, medical preparations with non-active ingredients, peptide/protein ingredients, etc. Treatment effect, low drug bioavailability and other issues, to achieve high drug loading capacity, maintain drug efficacy, and facilitate large-scale production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
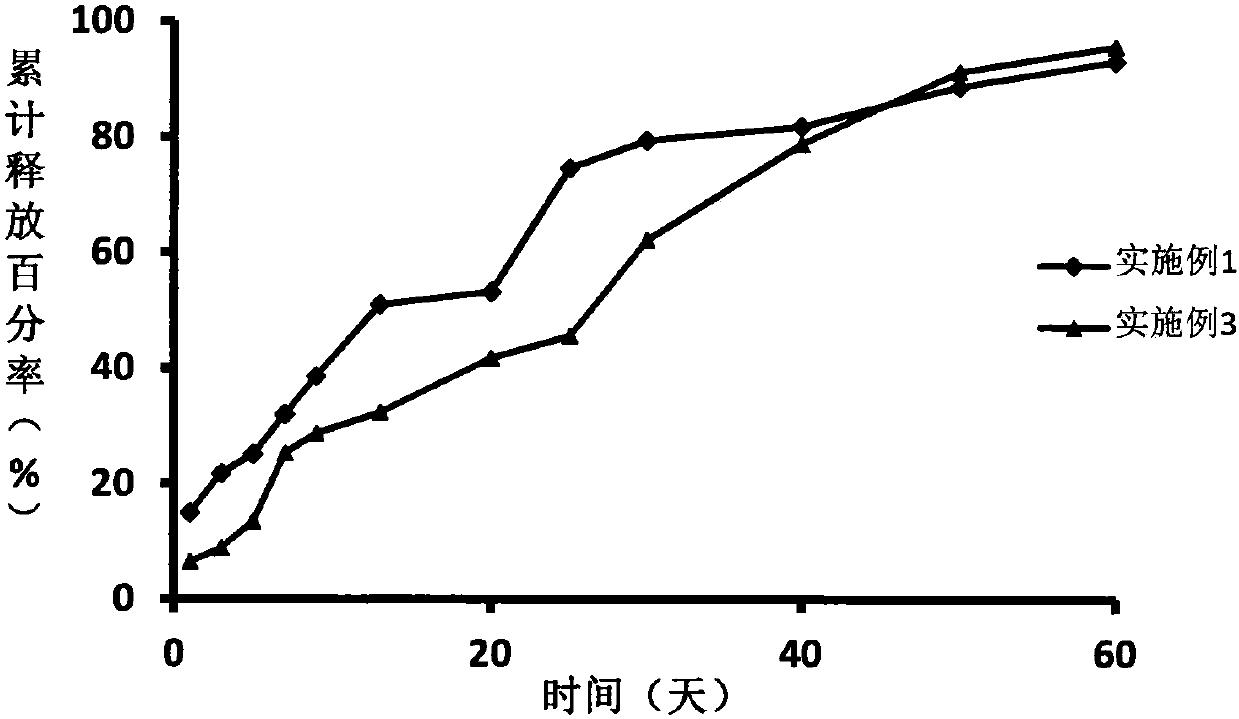
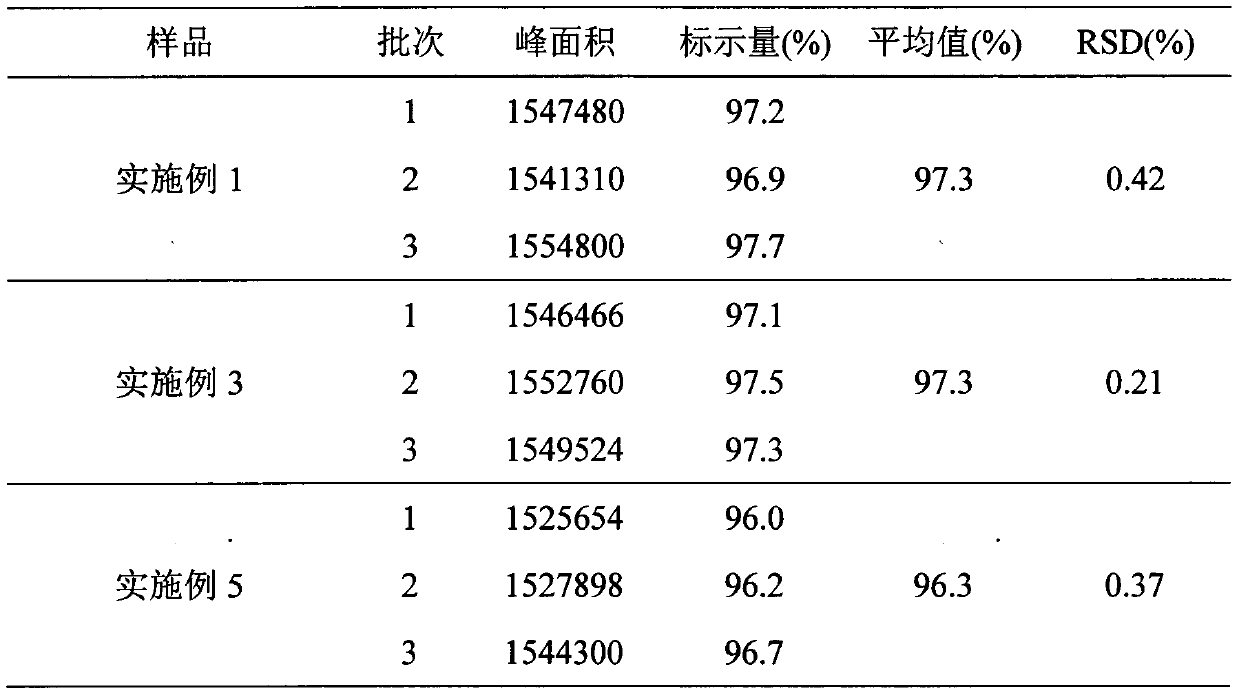
Embodiment 1
[0021] Take 24g of polylactide (Mw=15000Da), add it to an appropriate amount of benzyl benzyl ester, dissolve it ultrasonically, and configure it as a 10% polylactide solution, and then add 3.6g of degarelix acetate to the above solution , heated and stirred at constant temperature (temperature 30°C, 100 rpm) for 20 minutes to obtain a transparent and uniform drug-loaded sol system, which was sterilized by γ-ray irradiation to obtain degarelix acetate sustained-release implants for injection. Use a 1ml sterile syringe to draw an appropriate amount of gel, expel air bubbles, and seal it for use.
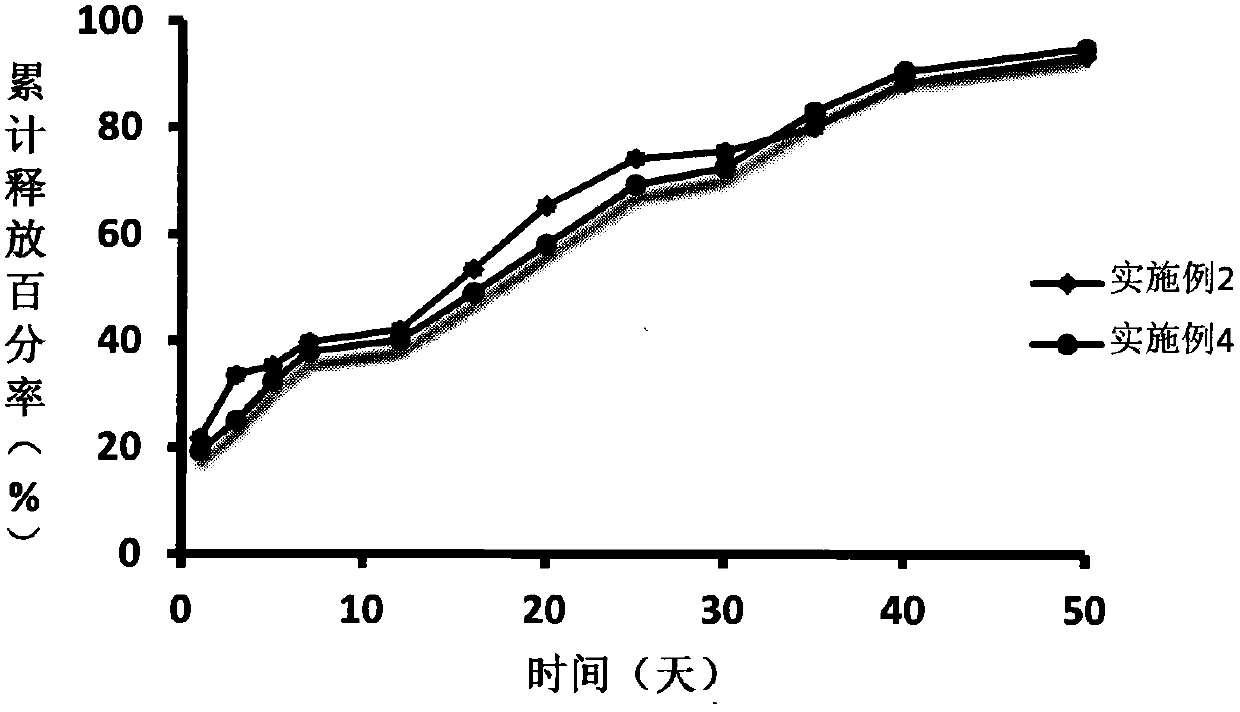
Embodiment 2
[0023] Take 6g of polylactide-caprolactone (Mw=8000Da), add it to an appropriate amount of N-methylpyrrolidone, dissolve it ultrasonically, and prepare a 25% polylactide-caprolactone solution, then add 3.6g of acetic acid Add the Rick raw material drug into the above solution, heat and stir at constant temperature (temperature 30°C, 100rpm) for 30 minutes to obtain a transparent and uniform drug-loaded sol system, and after γ-ray irradiation disinfection, the degarelix acetate injection delay release implants. Use a 1ml sterile syringe to draw an appropriate amount of gel, expel air bubbles, and seal it for use.
Embodiment 3
[0025] Take 4.5g of polylactic acid-glycolic acid (Mw=20000Da), add it to an appropriate amount of N-methylpyrrolidone, dissolve it ultrasonically, and configure it into a 18% polylactic acid-glycolic acid solution, and then add 1.8g of degarelix acetate raw material Add the drug into the above solution, heat and stir at a constant temperature (temperature 30°C, 100rpm) for 20 minutes to obtain a transparent and uniform drug-loaded sol system, which is sterilized by γ-ray irradiation to obtain degarelix acetate injection-type sustained-release implant agent. Use a 1ml sterile syringe to draw an appropriate amount of gel, expel air bubbles, and seal it for use.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com