Norovirus vaccine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
GI and GII Vaccine Formulation
[0051]Recombinant norovirus GI and GII VLPs expressed in Nicotiana benthamiana were obtained from Kentucky Bioprocessing (Owensboro, Ky.) as previously described [23] and used for powder formulations. Alternatively, recombinant Norovirus GI and GII VLPs expressed and purified from Sf9 insect cells using the baculovirus expression system were also used [18, 19].
[0052]The GelVac™ vaccine powders were made with a lyophilization-milling method. Liquid formulations were first prepared using a formulation that is comprised of the recombinant VLP in a solution with GelSite® polymer, povidone and lactose. They were then lyophilized. Following lyophilization, dried formulation contain 0.25% (w / w) GelSite®, 99% lactose and 0.05% povidone and 0 μg to 100 μg (based on ELISA data) of VLP per 20 mg of formulation, depending on the desired dose. The GI and GII VLPs were added together to the formulation to produce the multivalent powder or individually to produce the ...
example 2
Characterization of Vaccine Formulations
VLP Characterization
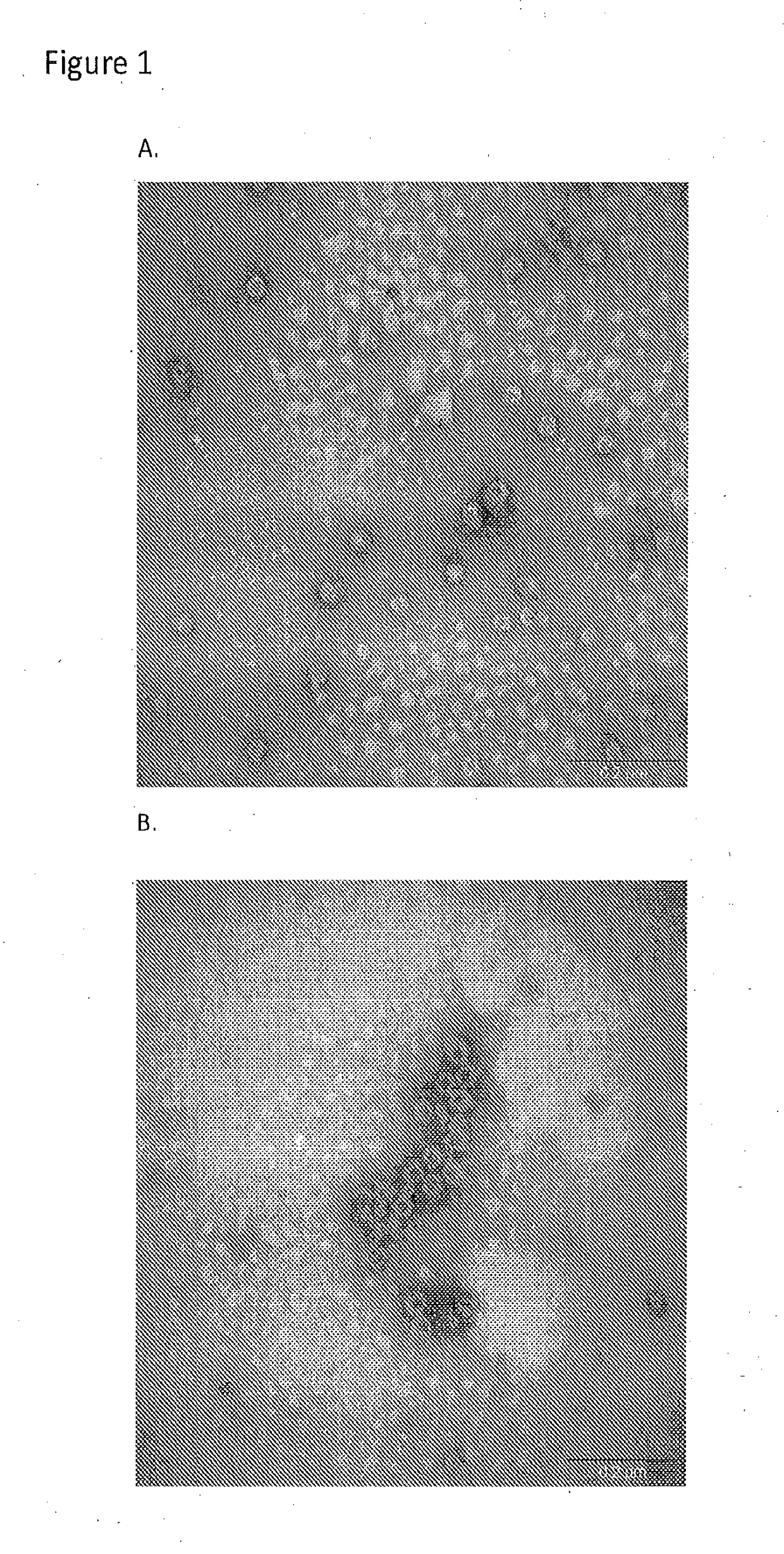
[0053]GI and GII VLP stocks were analyzed for the presence of intact VLPs by transmission electron microscopy prior to powder manufacturing. The results confirmed the presence of intact VLPs of the expected sizes (38 nm) for both GI and GII VLP stocks (FIG. 1).
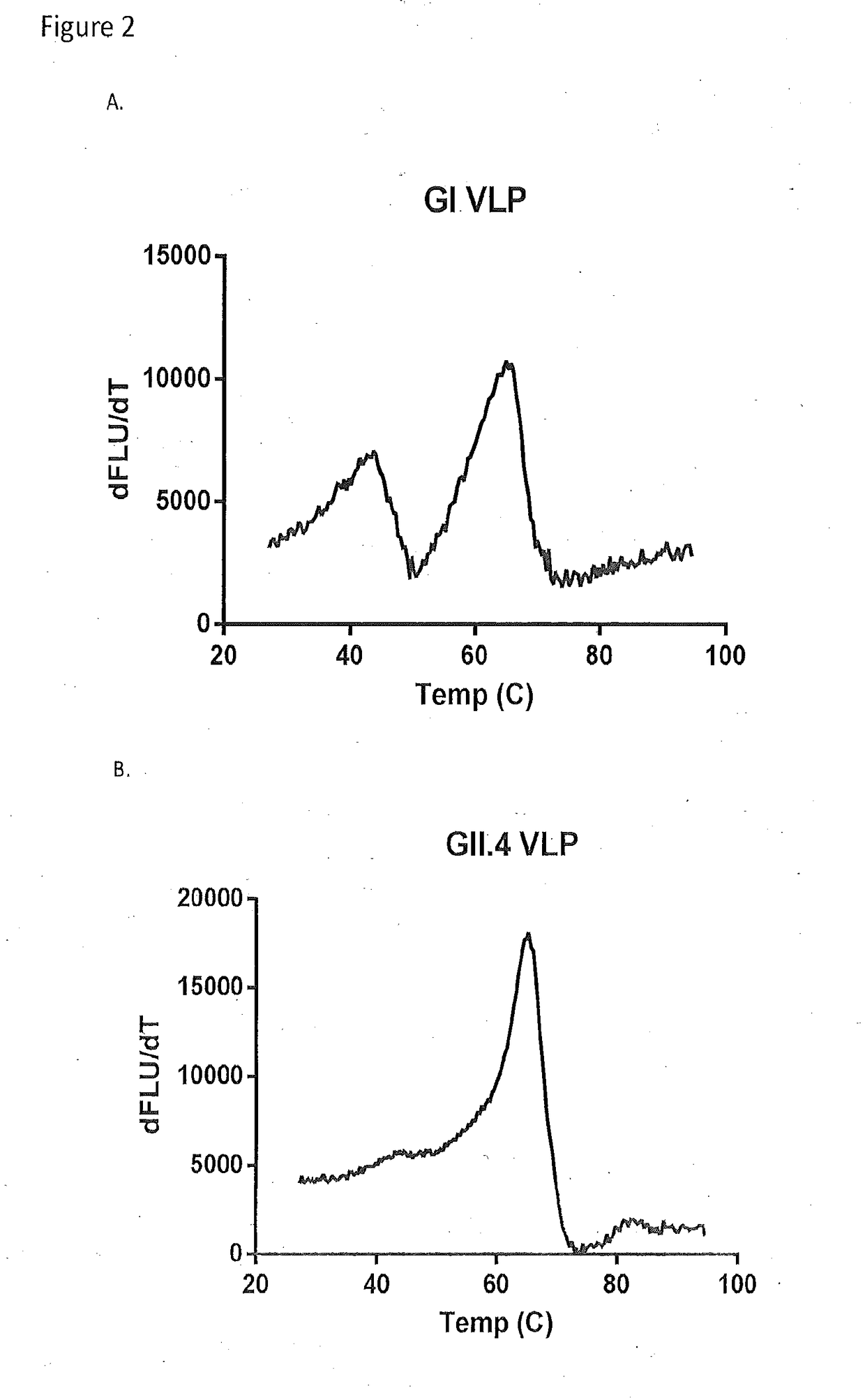
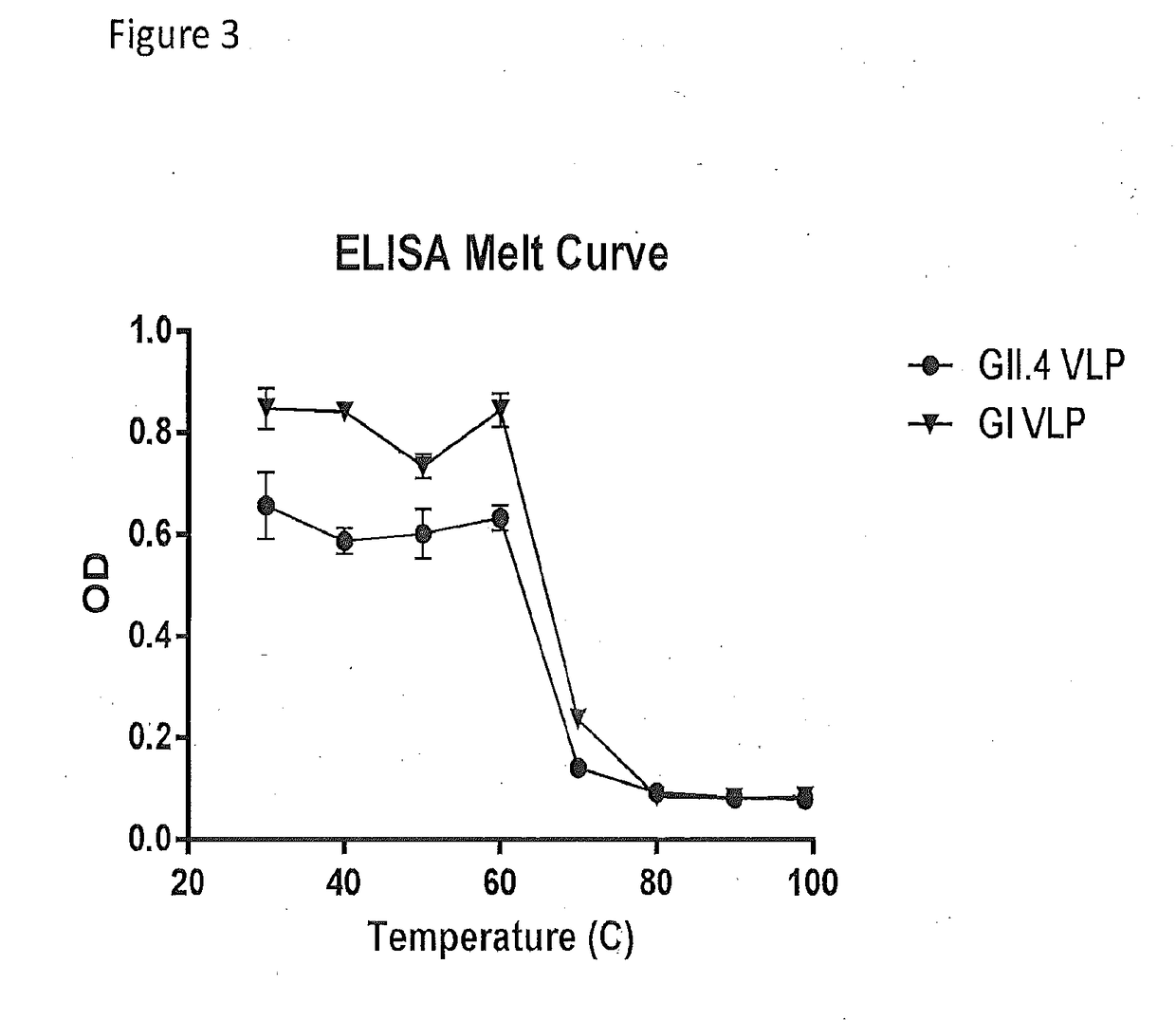
[0054]VLP stability was established by determining the melt temperature of norovirus VLPs with SYPRO Orange. Briefly, SYPRO Orange (Sigma-Aldrich, St. Louis, Mo.) was diluted in PBS to make a final 4× concentration of SYPRO Orange. Each VLP was diluted in 4×SYPRO Orange to a final concentration of 1 mg / mL. Each sample was then placed in a fluorescent thermocycler and was run through a 25′C-95′C gradient while reading the fluorescent signal. The derivative of the signal was determined by taking the difference between successive points in the fluorescent signal. The noise was reduced by using a 4-point moving average filter. Melt curve plots are shown for GI (FIG. 2A) a...
example 3
Immunogenicity of Vaccine Formulations
Immunogenicity of GelVac™ GI and GII Powders
[0060]The immunogenicity of a GelVac™ vaccine powder formulated with GI VLP has been reported previously [20]. To further these studies, antigen dose-dependent immune responses were investigated with GelVac™ vaccine powders with GI or GII VLPs in female (250 g) Hartley guinea pigs. Animals were dosed with varying amounts of norovirus GI or GII VLPs (Table 3) or with a multivalent GI / GII VLP vaccine (50 μg each of GI and GII VLP, twice on days 0 and 21.
TABLE 3Monovalent Vaccine Animal Experimental DesignMonovalent Guinea Pig StudiesGroup #nTotal Antigen per Vaccination (μg)*1410024503415445541640.1740*Animals were immunized with a total of 20 mg of powder via both nares. Each nare received 10 mg of powder or half of the total antigen dose.
[0061]The vaccine powders were administered intranasally using Aptar Unit Dose Spray (UDS) Devices (Aptar Pharma, Congers, N.Y.), one per nare with half of the total a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com